If a clinical trials of investigational medicinal product (CTIMP) application is submitted before 28 April 2026, it will follow the current approval timelines.
Any CTIMPs submitted on or after 28 April 2026 will follow the approvals process and timeframes set out in new clinical trials regulations. The following sections give guidance on how CTIMP applications will be processed from 28 April 2026.
This guidance sets out how the new regulations will change how Research Ethics Committees and the MHRA process applications.
It does not cover any other regulatory reviews CTIMPs may need, such as HRA and Health and Care Research Wales (HCRW) Approval.
This is because only the REC and MHRA review process for CTIMPs is within the remit of the clinical trials regulations and is directly impacted by the new legislation.
If changes are made to the processes and timelines for other reviews of CTIMPs, we’ll share separate guidance and information with you.
Initial application timeframes and process
Submission of applications
From 28 April 2026, if a CTIMP application is submitted to the Medicines and Healthcare products Regulatory Agency (MHRA) and Research Ethics Committee (REC) they will continue to be submitted by Combined Review.
In the submission, the sponsor will need to include all the documents the REC and MHRA need to review the application. The 2025 clinical trials regulations make changes to Schedule 3 (which listed the documents that applications to RECs and the MHRA must include) of the current 2004 regulations. While Schedule 3 has been updated, the documents that the REC expects to be submitted in the application will not change when the new regulations come into force.
For information on what documents RECs expect, read our current guidance for Combined Review applications and the 'amendment to schedule 3' section in the new clinical trials regulations.
For information on what documents the MHRA will expect, please see the MHRA guidance.
In exceptional circumstances, submitting separate applications to the MHRA and a REC may be acceptable. The expectation is that this will only be permitted where it would otherwise not be possible to submit a trial application in the UK.
If a sponsor believes they have an application that needs separate applications, they'll need to contact the MHRA at clintrialhelpline@mhra.gov.uk. In the email, they'll need to give details on the trial and explain why they believe separate applications are needed. Requests will be assessed on a case-by-case basis, and if agreed, instructions will be given on how to submit the application.
The REC will continue to offer a fast-track service for certain trials, with the service timelines remaining the same when the new clinical trials regulations come into force. The eligibility criteria for this service will not change. The eligibility criteria for the fast-track service, and a list of RECs that accept fast-track applications, is available on our website. If a sponsor has a trial that will use this service, they'll still need to book it directly with the REC.
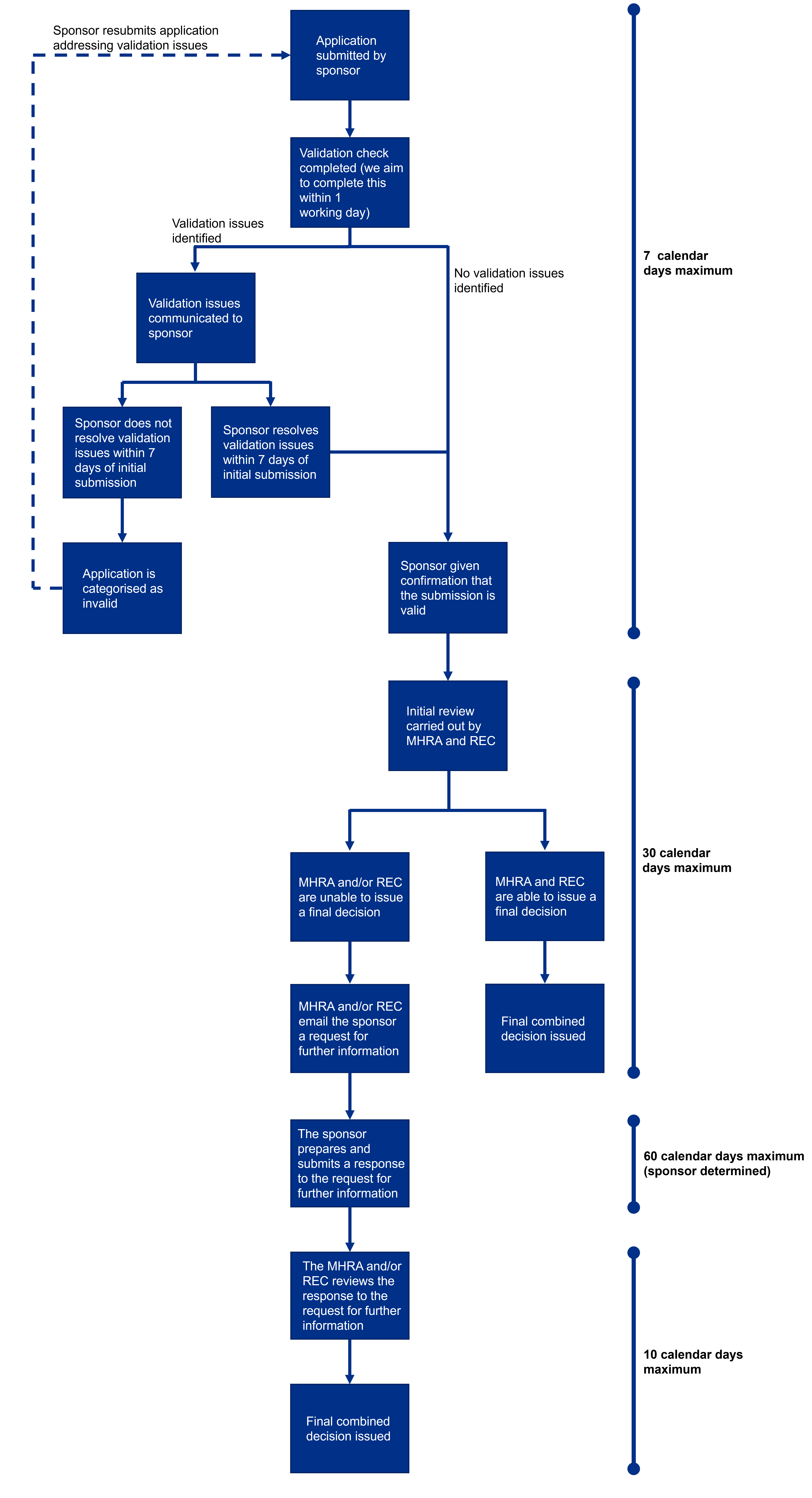
Validation of applications
When a sponsor submits an application, the MHRA and REC will carry out a check to confirm the submission is valid. We’ll aim to notify sponsors of the outcome of the validation check within 1 working day.
If we identify any issues preventing an application from being considered valid, we'll ask the sponsor to address these issues within 7 calendar days of the initial application submission. If these issues cannot be resolved within 7 days, the submission will be invalid. The sponsor will need to resubmit their application, making sure all issues have been addressed. All submissions will be confirmed as either valid or invalid within 7 calendar days of the submission.
For an application to be valid, it must include all required documents. PDF documents must be created directly from Microsoft Word or undergo Adobe Acrobat optical character recognition at the time of creation.
Initial review of trial applications
Once an application is valid, the MHRA and REC will conduct an initial review. This includes the application being discussed at a full meeting of the REC. The MHRA and REC will complete the initial review as quickly as possible, and the outcome will be shared with the sponsor, and relevant individuals listed in the application, by email within a maximum of 30 calendar days of the application being confirmed as valid.
When booking an application to a REC, the sponsor will be offered a selection of possible REC meeting dates. If they choose the next available REC meeting, the start point for the 30 day period (for both the REC and MHRA review to be completed) will be when the application is confirmed as valid.
If the sponsor does not choose the next available REC meeting, the 30 day period will begin 7 days before the chosen REC meeting, as long as the sponsor submits a valid application by that point. If a valid application has not been received, the application may be withdrawn, and the sponsor will need to resubmit for review at a different REC meeting.
Initial review outcomes
There are three possible outcomes from the initial REC review:
- favourable opinion
- favourable opinion subject to conditions
- unable to issue a favourable opinion and requests further information
The REC opinion and the MHRA decision together will form the outcome of the initial review. If the REC and the MHRA are unable to approve the trial at this stage, a request for further information will be issued. This may be from the REC, the MHRA, or both.
Requests for further information
If the REC is unable to issue a favourable opinion after the initial review, it will email the sponsor to request further information. The request will be sent as soon as it’s been confirmed by the REC. This means the sponsor will be able to receive and consider feedback from the REC as soon as it’s available.
It should be noted, however, that the sponsor will still need to wait for the full request for further information to be issued in IRAS before the sponsor responds to any points they receive individually from the MHRA or REC. This is because the full request for further information will have the final consolidated feedback, including any queries or issues, from both the MHRA and REC.
When the new clinical trials regulations come into force, sponsors will have up to 60 calendar days from when the request is sent to respond. Sponsors can respond as early as they would like within these 60 days. The sponsor should not include any changes to documents or information that are not in response to the points raised when they respond to a request for further information.
If sponsors need longer than 60 days to respond, they can ask for an extension. Sponsors can do this by contacting the MHRA at clintrialhelpline@mhra.gov.uk or, if the points raised only relate to the REC review, by contacting the REC directly. In their request, the sponsor will need to explain why they need an extension and when they expect to respond. If an extension is agreed, the REC and MHRA will make each other aware of this.
If a request for an extension is not made (or agreed) and the sponsor does not submit a response within 60 calendar days, the MHRA will treat the application as rejected, and the REC will issue an unfavourable opinion.
Reviewing a response to a request for further information
If the REC and/or MHRA send a request for further information, an outcome will be given within 10 calendar days once the sponsor has responded to the request. If the response is incomplete, or does not address the matters raised, the REC or MHRA will contact the sponsor and ask them to submit a complete response to the request for further information.
The 10 day timeframe will not begin until a complete response to all issues raised by both the REC and MHRA (as relevant) is received. Once a complete response is received, the MHRA and REC will review the response and provide the outcome as quickly as possible.
There are three possible outcomes from REC reviewing a response to a request for further information. These are:
- favourable opinion
- favourable opinion subject to conditions
- unfavourable opinion
If the MHRA issues grounds for non-approval and/or the REC identifies ethical issues that mean an unfavourable opinion is given, the sponsor will still be able to appeal the decision. They can do this by contacting us at appeals@hra.nhs.uk within 28 calendar days of receiving the outcome. In their appeal, the sponsor must explain why they disagree with the outcome.
Independent expert advice
For some applications, the REC or MHRA may need independent expert advice from a specialist group or committee.
For information on when independent expert advice may be needed, read the MHRA's guidance. Their guidance also explains what a sponsor should do if they suspect their application may need independent expert advice.
When independent expert advice is needed, it will be sought during the initial review of an application and/or after receiving a response to a request for further information. The timeframes for the approval process may be extended to allow for the advice to be requested and received.
If independent expert advice is sought:
- during the initial review stage for your application, the 30 day timeframe may be extended by up to an additional 90 calendar days (bringing the maximum timeframe to 120 calendar days)
- following a response to a request for further information being received, the 10 day timeframe may be extended by up to an additional 30 calendar days (bringing the maximum timeframe to 40 calendar days)
- following a response to a request for further information being received and an advanced therapy medicinal product (ATMP) is involved in the trial, the 10 day timeframe may be extended by up to an additional 60 calendar days (bringing the maximum time frame to 70 calendar days)
In all cases, we’ll make sure it proceeds as quickly as possible to avoid unnecessary delays in sponsors receiving the outcome.
If the REC or MHRA determine that they need to seek independent expert advice, they'll notify the sponsor, ask the sponsor for any additional information needed, and let the sponsor know when they can expect to receive the outcome.
Timeline for xenogenic cell therapy applications
If a sponsor submits an application involving xenogenic cell therapy, the same review process will be followed. Both the MHRA and REC will review the application as efficiently as possible, as they would any other application. However, the above timeframes will not apply. This means that outcomes and decisions can be issued at any point after an application is received and validated.