A Phase 1 trial means a clinical trial to study the pharmacology of an investigational medicinal product when administered to humans, where the sponsor and investigator have no knowledge of any evidence that the product has effects likely to be beneficial to the trial participants.
Automatic deferral period
If a sponsor submits a Phase 1 clinical trials of investigational medicinal product (CTIMP) application only involving healthy volunteers from 28 April 2026, it will automatically be deferred for all transparency requirements.
This means a sponsor will not be required to include a written request for a deferral as part of the application. It will be applied automatically upon receipt of a Phase 1 CTIMP only involving healthy volunteers. Confirmation that a deferral is in place will be provided as part of the final approval given to a trial.
Phase 1 healthy volunteer CTIMPs submitted and approved before 28 April 2026 may be given an automatic deferral. However, this depends on whether the end of trial has been declared by this date and whether a deferral is already in place by that point.
The following figure summarises what will happen in each scenario.
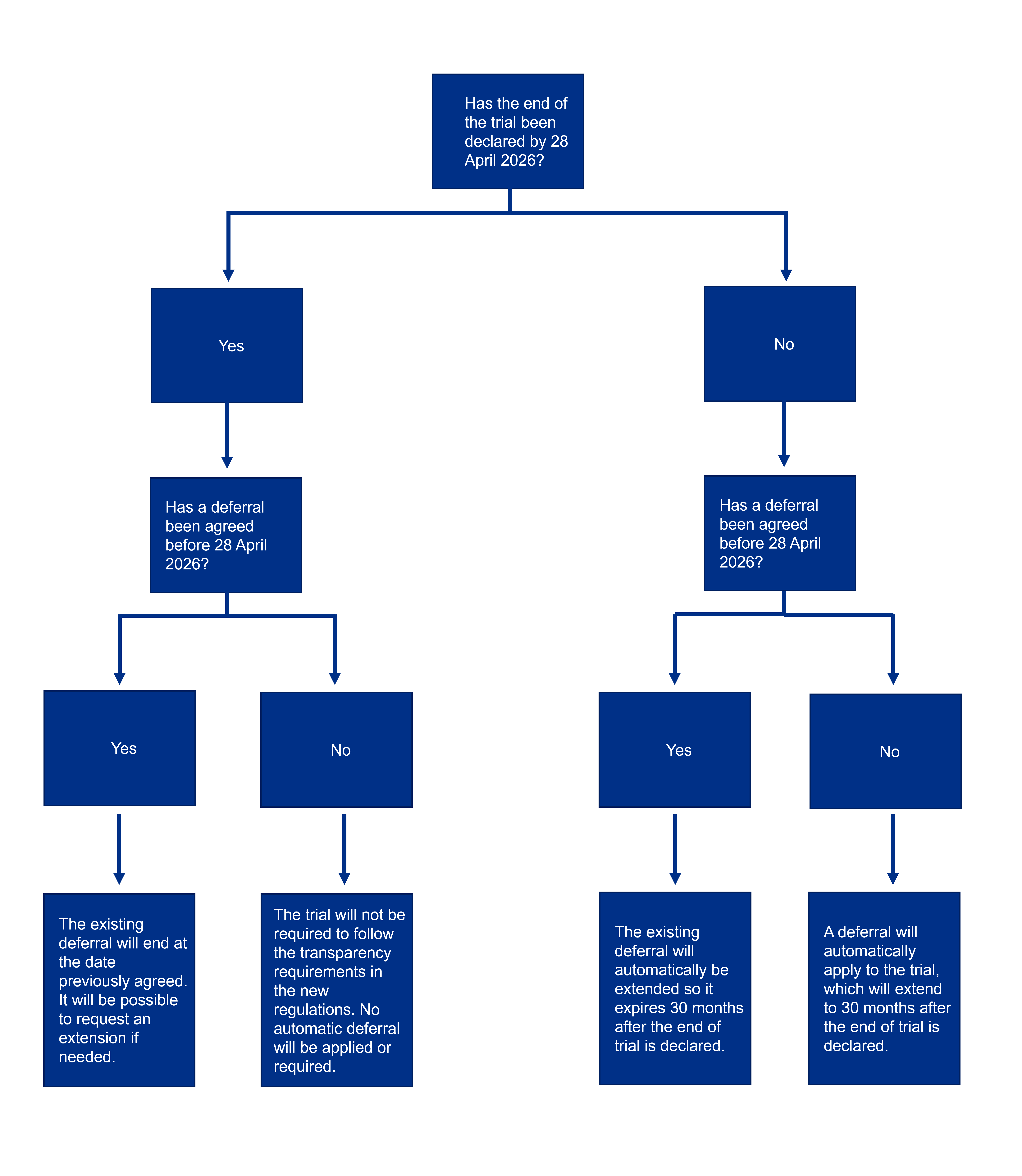
Requirements for Phase 1 trials with automatic deferrals
If a trial has an automatic deferral in place, the sponsor will not have to:
- register the trial in full until 30 months after the trial has ended, however the sponsor must still publish a minimal record on a publicly accessible registry (a minimal record can be published with ISRCTN)
- publish the summary results of the study until 30 months after the trial has ended
- offer to share results with participants during the deferral period
Where a trial qualifies for the automatic deferral, the sponsor will still need to carry out the activity in the 'requirements once a deferral is agreed' section of our deferrals guidance.
It’s still important for sponsors to thank participants or relevant people and provide them with a timeframe for receiving results, even if a deferral is in place.
Although all Phase 1 CTIMPs involving only healthy volunteers submitted from 28 April 2026 will be provided with an automatic deferral, it’s up to the sponsor to decide whether to use this deferral or not.
This means that if the sponsor chooses to register the study, publish summary results, and/or offer to share findings with participants during the automatic deferral period, they can do so.
Extending the deferral for publishing and offering to share summary results with participants beyond the automatic deferral period
We acknowledge that, for Phase 1 healthy volunteer trials, legitimate concerns around commercial confidentiality will often continue beyond the initial automatic deferral period, particularly in relation to the publication of summary results and the offer to share them with participants.
To address these concerns, the sponsor will be able to request, in the cover letter accompanying their initial clinical trial application, 2 additional consecutive 30 month deferral periods beyond the automatic deferral period to cover publication of the summary of results and offering to share results with participants.
Where a sponsor requests these extensions on the grounds of commercial confidentiality, the request will normally be granted unless there are exceptional circumstances. Sponsors will only be able to request 2 additional 30 month extensions as part of the initial clinical trial application. After the trial is approved sponsors will only be able to extend their automatic deferral by following the process for extending deferrals as outlined in our deferrals guidance.
This will mean that a sponsor of a Phase 1 healthy volunteer trial will not be required to publish the summary of results or offer to share results with participants until 90 months (7.5 years) after the end of the trial.
If the sponsor reaches the end of this 90 month period and wants to defer further, they will be able to request 1 additional 30 month extension by following the process outlined in our deferrals guidance.
Where a sponsor requests this extension on the grounds of commercial confidentiality, the request will normally be granted unless there are exceptional circumstances, for example, if the trial is no longer commercially confidential because summary results have already been made public.
In total, sponsors will be able to defer up to a maximum of 10 years from the end of the trial. After 10 years, the sponsor will have to comply with the transparency requirements.
We do not expect requests for the additional 60 month extension, or any deferral requests extending beyond 30 months after the end of the trial, to include the registration requirement, as these requests will not normally be approved except in exceptional circumstances.
Deferrals for Phase 1 CTIMPs involving patients
If a trial is a Phase 1 CTIMP involving patients it will not automatically be deferred, even if it will involve both healthy volunteers and patients. Sponsors of these trials will be able to request an initial deferral of the transparency requirements by following the process in our deferrals guidance.
Although there is no automatic deferral, sponsors of phase 1 trials involving patients will be able to request 2 additional 30 month extensions to the deferral to publish summary results and offering to share results with participants when requesting an initial deferral.
Sponsors will only be able to request this as part of their initial clinical trial application. Once a trial is approved, sponsors will only be able to extend their initial deferral by following the process described in our deferrals guidance.
If this is requested and agreed, the sponsor will not need to complete these activities until 90 months (7.5 years) after the end of the trial. However, we still recommend that sponsors of Phase 1 trials involving patients thank the participants for taking part in their trial and explain to participants, and any relevant people, at what point they might receive the results of the study.