Transforming the UK's clinical trial landscape
The UK's clinical trial regulations are changing.
The Health Research Authority (HRA) has been working in partnership with the Medicines and Healthcare products Regulatory Agency (MHRA) to amend the Medicines for Human Use (Clinical Trials) Regulations 2004.
Our goal is to create a modern, streamlined regulatory environment that maintains high standards of participant safety while accelerating access to innovative treatments.
Feedback from a public consultation in 2022 helped shape proposals to improve how clinical trials of investigational medicinal products (CTIMPs) are run in the UK. These changes aim to make the UK one of the best places in the world to conduct clinical research.
The updated Medicines for Human Use (Clinical Trials) (Amendment) Regulations 2025 have now been approved.
Following a 12-month implementation period, the regulations will come into force on 28 April 2026.
This page brings together everything you need to know during the implementation period and beyond.
Webinar - Clinical Trials Regulations: Countdown to implementation
The Health Research Authority (HRA) and Medicines and Healthcare products Regulatory Agency (MHRA) are holding a webinar about the changes to clinical trials regulations that come into effect on 28 April 2026.
The Clinical Trials Regulations: Countdown to Implementation webinar takes place online on Thursday 12 March from 2pm to 4pm (GMT).
We’ll cover the key changes in the updated regulations, the guidance we’ve published to support you through the transition, and our panel of HRA and MHRA experts will be on hand to answer your questions.
The webinar will be recorded, so if you can’t join, the recording will be made available soon after.
If you have any queries about the webinar, please email conferences@mhra.gov.uk
Who these changes affect
These updated regulations will impact everyone involved in CTIMPs in the UK:
- researchers and sponsors
- Research Ethics Committees (RECs)
- trial participants and the public
- healthcare professionals
Why these changes matter
The reforms will:
- align the process for the MHRA and RECs to review and approve applications and substantial modifications
- enhance transparency and accountability for research findings
- reduce unnecessary burdens while upholding ethical and safety standards
Together, these improvements will strengthen the UK's position as a global leader in clinical research.
Key changes in the regulations
The following areas of the regulations have been updated.
New and updated definitions
The term ‘amendment’ to describe changes to approved trials will be replaced with ‘modification’. This term is already used by European Union nations to describe changes to clinical trials of investigational medicinal products (CTIMPs). This change will make sure there is better alignment between the UK and the international research community.
From 28 April 2026, modifications will be categorised as ‘substantial modifications’, ‘modification of an important detail’ or ‘minor modifications’.
The updated regulations introduce new definitions including ‘non-investigational medicinal product’, ‘notifiable trial’ and ‘public registry’.
There are also changes to existing definitions including the removal of the term ‘authorised health care professional’. Instead, the updated regulations say that chief investigators and investigators should be a health care professional as defined in the amended legislation.
The updated regulations also replace the term ‘subject’ with ‘participant’ and ‘trial site’ with ‘trial location’.
The approvals process for clinical trials
Combined Review – the system that lets researchers apply for ethics and regulatory approval in one go – and a new streamlined notification scheme for some clinical trial initial applications and amendments, will be written into law as part of the updates.
The updated regulations will result in changes to how Research Ethics Committees (RECs) and the MHRA process applications and amendments (modifications from 28 April 2026) for CTIMPs, including for Phase 1 healthy volunteer trials.
Under the amended regulations, the MHRA and RECs will be able to request that sponsors modify their trials in certain circumstances.
The legislation also sets the expectation that all CTIMPs should recruit their first participant in the UK within 2 years of the trials being approved, with extensions available. If there is no recruitment within 2 years and an extension has not been granted, the approval will lapse.
Transparency
For the first time in the UK, it will be a legal requirement to:
- register clinical trials involving medicines in a public registry
- publish a summary of trial results within 12 months of completion
- offer to share a summary of results with participants (or other relevant people) in a way they can easily understand
Provisions for deferrals in specific circumstances, such as phase 1 trials involving healthy volunteers, will be available.
Research Ethics Committees (RECs)
Changes to RECs will improve flexibility while aligning with international good clinical practice (ICH-GCP E6). When the new regulations come into force RECs will:
- need at least five members that collectively possess the qualifications and experience to review and evaluate any proposed trial's scientific, medical, and ethical aspects
- still be expected to have an appointed Chair
- include one lay member
Simplified arrangements for consent in clinical trials
The new clinical trials regulations will offer sponsors of low intervention clinical trials (studies using authorised medicines in routine healthcare) the option to use simplified arrangements for seeking and evidencing informed consent.
As part of this work, we’ve established an advisory group, made up of a range of people with appropriate expertise, skills and experience, to help us develop a set of principles that:
- provides guidance for sponsors and researchers looking to use simplified arrangements
- sets HRA expectations on the use of simplified arrangements that maintain people’s trust
- supports REC members to evaluate the ethical considerations of using simplified arrangements for seeking and evidencing consent
Read more about the advisory group and its members.
Guidance instead of legislation
Some areas will be supported through detailed guidance rather than new legal requirements.
We are leading work on guidance to support best practices in diversity and inclusion, and public involvement in clinical trials.
Diversity and inclusion in clinical trials
Including a wider range of participants ensures trial findings reflect the entire population's needs.
Our Public Perceptions of Research report found that:
- 88% believe trials should involve a diverse group of participants.
- 70% support this even if it increases costs
- 74% are in favour even if it extends timelines
We've developed a draft set of questions and supporting guidance jointly with the Medicines and Healthcare products Regulatory Agency (MHRA) for researchers to consider when they design clinical trials and clinical investigations. The answers to these questions will form the basis of an Inclusion and Diversity Plan.
This will help make sure clinical research is designed to include people who could be impacted by the findings, and that people underserved by research are not overlooked.
Following an informal consultation on the draft questions and supporting guidance in 2024, we're now running a pilot in which sponsors and researchers are invited to submit a plan as part of their IRAS application.
The pilot, which opened in May 2025, will be closing soon. We'll update you on our progress and next steps in early 2026 once we've reviewed the feedback.
Public involvement
Involving the public in designing, delivering, and reporting clinical trials leads to better outcomes and builds trust.
We're updating our website to:
- provide clear expectations for public involvement in health and social care research, including all phases of clinical trials
- point to the wide range of resources available across the sector
- include specific recommendations for Phase 1 healthy volunteer trials
The updates will help researchers meaningfully involve the public in all clinical trials.
Timeline of key changes
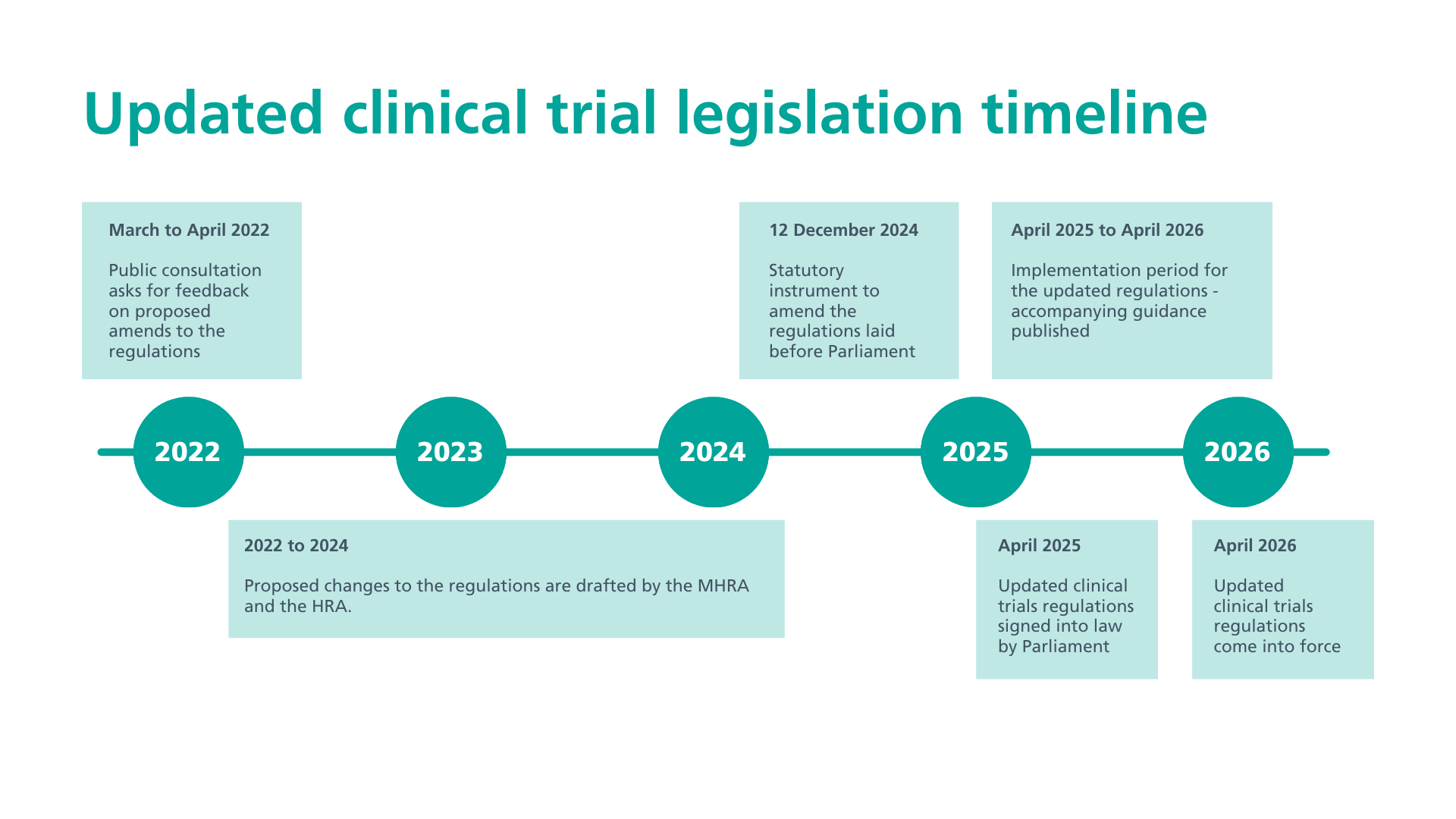
Past milestones
- March to April 2022 - public consultation
- December 2024 - Statutory Instrument laid before Parliament
- February 2025 - approved by Westminster Parliament and House of Lords
- April 2025 - approval from the Northern Ireland Assembly and final ministerial sign off
- June 2025 - new guidance is published to accompany the new regulations
- 1 October 2025 - final guidance to accompany the regulations is published
- 28 October 2025 - 6 months until clinical trials regulations come into force
What's next
- 28 April 2026 - implementation of the amended regulations
Support for researchers and sponsors
We're working closely with our stakeholders to ensure there is a smooth transition for the updated regulations.
To help with this we'll be producing:
- guidance – new resources will be published throughout 2025 (see guidance section below)
- training – upcoming Clinical Trials Regulations: Countdown to implementation webinar on Thursday 12 March
- news and blogs – sharing the latest updates on our website and via our Clinical Trials update
New guidance to accompany clinical trials regulations
We’ve published our final guidance to help prepare researchers and sponsors for the changes that will happen when the new clinical trials regulations come into force in April 2026.
Thank you to everyone who shared their thoughts and comments on previous versions of the guidance.
The guidance outlines the review and assessment processes for CTIMPs by RECs and the MHRA.
Our guidance covers the following updates to the regulations which have been a key focus for the HRA:
- definitions and terminology
- pharmacovigilance
- the approvals process for clinical trials
- Research Ethics Committees that review clinical trials
- simplified arrangements for consent in clinical trials
- research transparency requirements for clinical trials
The guidance does not cover changes to other reviews of CTIMPs, for example study wide review (HRA and HCRW Approval) and reviews of non-CTIMP applications. This is because the amended regulations do not apply to these types of studies.
Read more about the final guidance in our news story.
Find out more about the journey over the last year to develop our guidance and the work behind the scenes in this blog post from Catherine Blewett, Senior Development Manager, and Chris Cole, Guidance and Advice Manager.
MHRA guidance
The MHRA has also published separate guidance to accompany the amended regulations which you can read on their clinical trials hub.
Changes to non-CTIMP studies
We're introducing changes to how other types of health and social care research (non-CTIMPs) are processed and managed to align with the updated clinical trials regulations.
This will promote consistency across all UK clinical research and will streamline the regulatory process, making it simpler for sponsors and researchers to obtain approvals.
The HRA has published new guidance to help sponsors and researchers prepare for the changes to non-CTIMPs.
Useful links
- MHRA – Clinical Trials Regulation
- NIHR – Supporting Research in the UK
- EU Clinical Trials Regulation (CTR)
Sign up for updates on the clinical trial regulations

We send out regular email updates about the amended clinical trials regulations in the UK.
These emails include updates on the different areas the HRA is leading on, and support for researchers and sponsors.
You can take a look back at previous editions on our clinical trials update webpage.
Clinical trials regulations news
Visit our clinical trials regulations news and updates page for a round up of our work on the regulations.