Our strategy 2025-28
Boosting research that improves health and grows the economy
Download our strategy
Scroll down to read more, or download a PDF of our strategy
Foreword by Matt Westmore, Chief Executive and Neelam Patel, Chair
We are at a pivotal moment for health and social care research in the UK. The government has made clear its ambition to make the UK the best place in the world for medical research.
The Prime Minister’s recent commitment to ‘turbocharge’ clinical trials, backed by major investment in digital infrastructure, signals a bold new chapter for our sector. This is part of a broader mission to deliver an NHS fit for the future, economic growth, address inequalities, improve patient care and reshape our public services for the future.
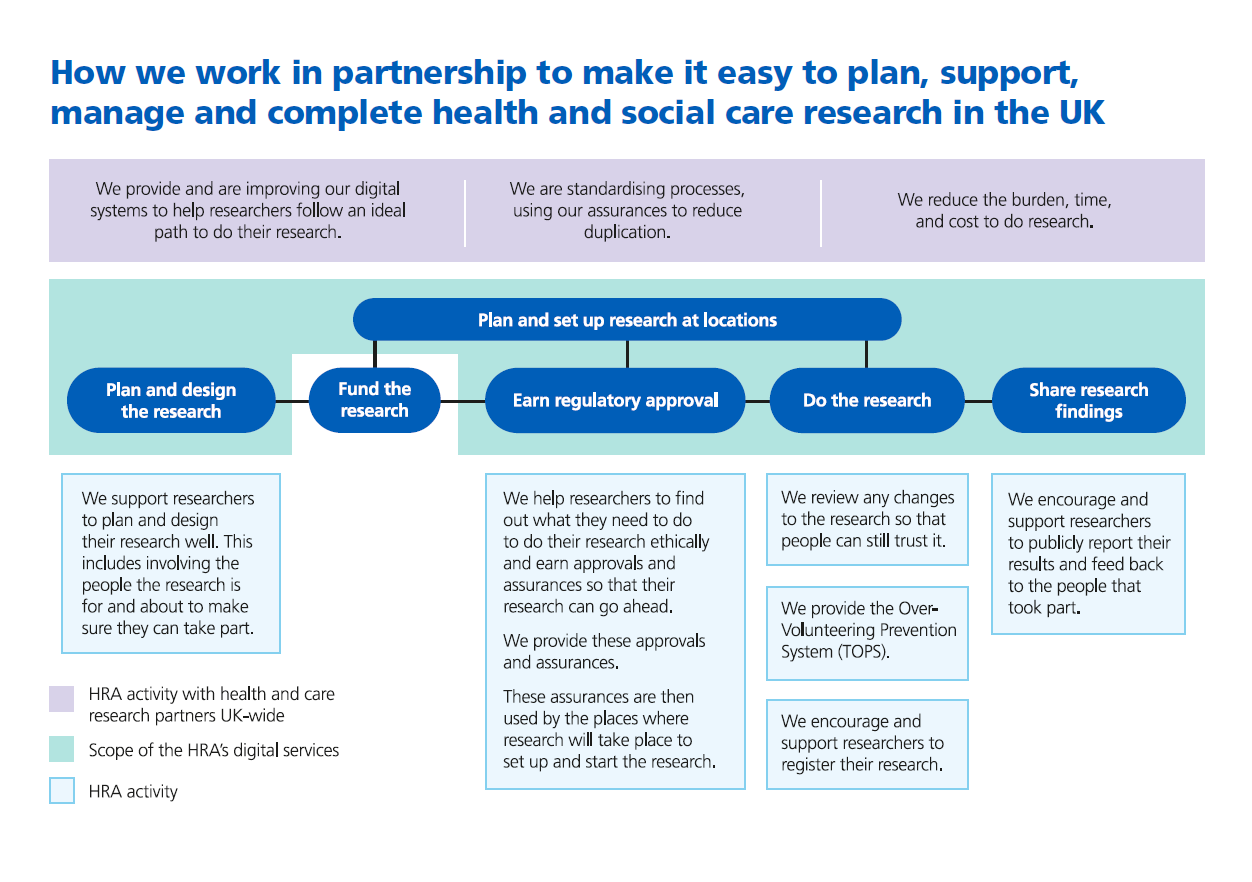
The Health Research Authority (HRA) was created to transform the regulation and governance of the health and social care research in the UK. We do this through the services we run - providing fast, trustworthy approvals and assurances - and by connecting the end-to-end research system so that studies can be planned, set up, delivered, and completed more efficiently and effectively.
Over the next three years, we will turbocharge this work. We will modernise our digital services to support a world-leading research environment, aligned with the government’s vision for smarter, more productive public services, as we make it easier for researchers and the NHS to do research. We will continue to set and uphold high standards for research, working with our partners to ensure that research is done well - with and for everyone.
Trust is the golden thread that runs through all of this. Without trust, the public won’t want to take part in research or allow their data to be used. Without trust, patients and clinicians won’t want to use the fruits of research. People will not be confident that their charity donations can make a difference. And without trust, companies and researchers won’t choose the UK as a place to invest and innovate. Building and maintaining that trust is not optional - it is fundamental.
We know that trust is not yet universal. Some communities - particularly Black people - are significantly less confident about taking part in research than White and Asian people. At the same time, industry stakeholders often view the UK as a challenging place to deliver research, citing inefficiencies and fragmented processes. If we are to lead globally, we must address both trust and system performance head-on.
The UK is well-placed to be the leading life sciences economy in Europe. We have a strong science base, a diverse population and a national health service. With the right systems, standards, and safeguards in place, we can unlock the full potential of data, artificial intelligence (AI), and new technologies to transform care - from hospital to home, analogue to digital, treatment to prevention.
This strategy sets out how the HRA, working with our partners, will contribute to this national mission. It is a call to action - to researchers, to the public, and to our partners across the system. Together, we can create a research environment that is faster, fairer and more impactful. Most importantly, we can change and improve lives through a vibrant UK health and social care research sector.
We are excited by what this strategy represents, and look forward to making this a reality with you.

Chief ExecutiveMatt Westmore

ChairNeelam Patel
How we work in partnership to make it easy to plan, support, manage and complete health and social care research in the UK
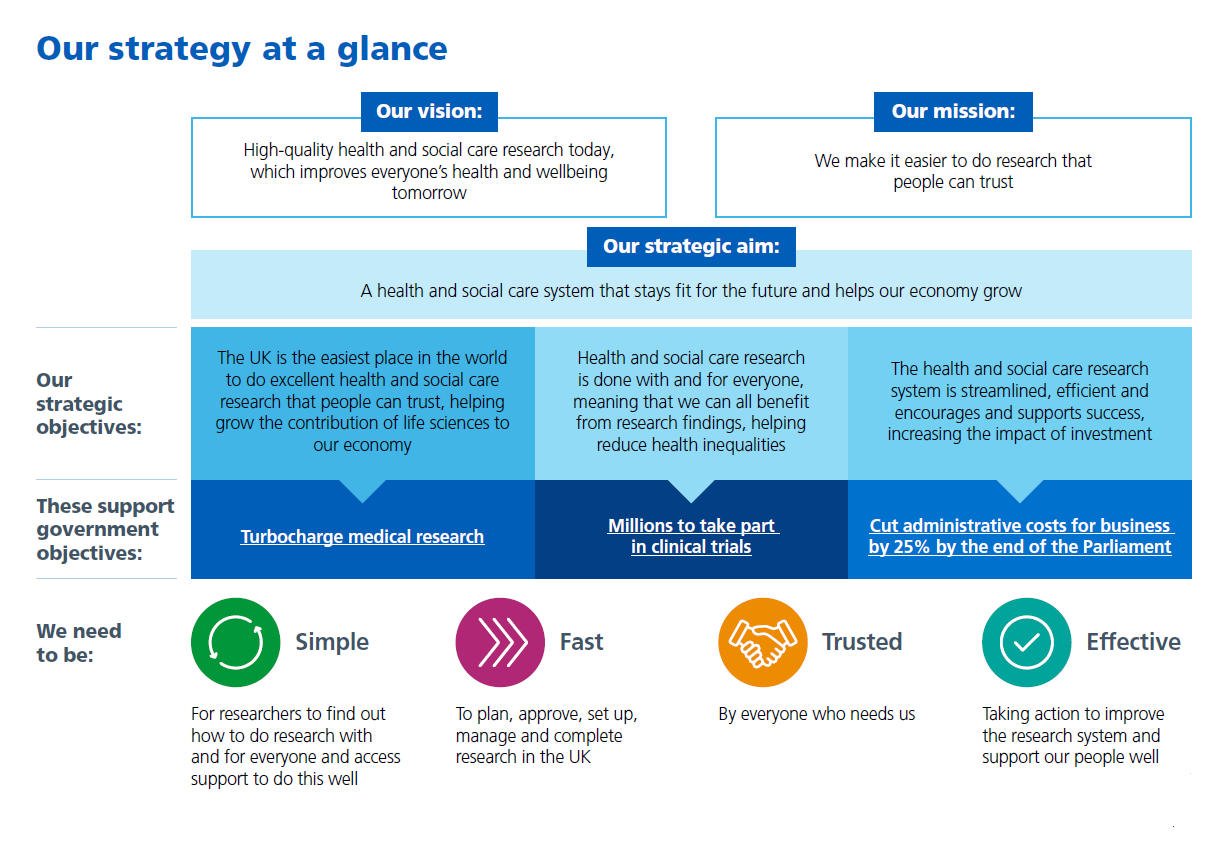
Our strategy at a glance
Key outcomes that you will see
Simple
- it is easier to do research across different settings in the UK, broadening access to research
- research is more transparent
- more research meaningfully involves the public to inform the way it is done
- there is greater representation in, and access to, research so that people from all backgrounds across the UK can take part in research relevant to them
- researchers can do research in new ways and using new technologies such as AI to improve care in a way that people can trust
Fast
- it is faster and easier to use our digital services to plan, approve, set up, manage and complete research in the UK
- setting up research is more efficient with simple steps and no duplication
- we provide approvals in time to support the set up of clinical trials in 150 days
Trusted
- people can understand, value and trust us and the way that we work
- our assurances are understood, valued and trusted, not unnecessarily repeated to set up research
- our digital services are secure
- people know when and how to raise questions and concerns with us and we are clear we will act on them
Effective
- we are playing our part in reducing government running costs
- we are using AI well
- we have safe, scalable and secure digital infrastructure
- our staff have a high level of engagement
- our community of volunteers and public contributors have a good experience with us and can see the difference they make
How we do this
We give approvals and assurances, working in partnership UK-wide to provide a world-class regulatory system.
We are part of modern, digital government.
Anyone can understand what we do and why it matters to them and be confident in our decisions.
We set shared expectations for how to do research and encourage compliance with these.
Our people can be their best.
We are efficient and take action to increase productivity.

How this strategy helps us achieve our vision and mission
The Health Research Authority is part of the health and social care research sector that is focused on improving everyone’s health and wellbeing.
This is important because research findings can improve everybody’s care faster if more health and social care research is taking place that a wider diversity of people are confident and able to take part in. Getting this right will improve the health and wellbeing of everybody in the UK and contribute to economic growth.
Our last strategy set ambitious objectives to accelerate the process of doing research and change the way that research is done so that it includes everyone.
This strategy builds on that work, taking the opportunity to align it with government-wide missions to make change happen faster.
The UK government has set out five missions as part of its plan for change. Our work directly supports the missions for health and growth.
At the heart of our work are people. People who could benefit from research findings improving their care, helping them to have a better quality of life and play a more active role in society.
The UK government’s health mission sets out to address the main underlying drivers of ill-health and tackle persistent inequalities in health to put the NHS on a sustainable footing for the future. Research will be key to doing this well. Research that has been done with and for everyone so that the whole population can benefit from its findings will do this best. That is why our work to ensure that research is done in a way that people can trust and includes everyone, is important to build a health service fit for the future.
The UK is a great place to do high quality health and social care research - with a diverse population, strong science base and a national health service. Attracting more research to happen here will benefit both people in the UK and help grow our economy.
That is why our work to make it easier to do research is important to the UK government’s growth mission to kickstart the UK’s economic growth.
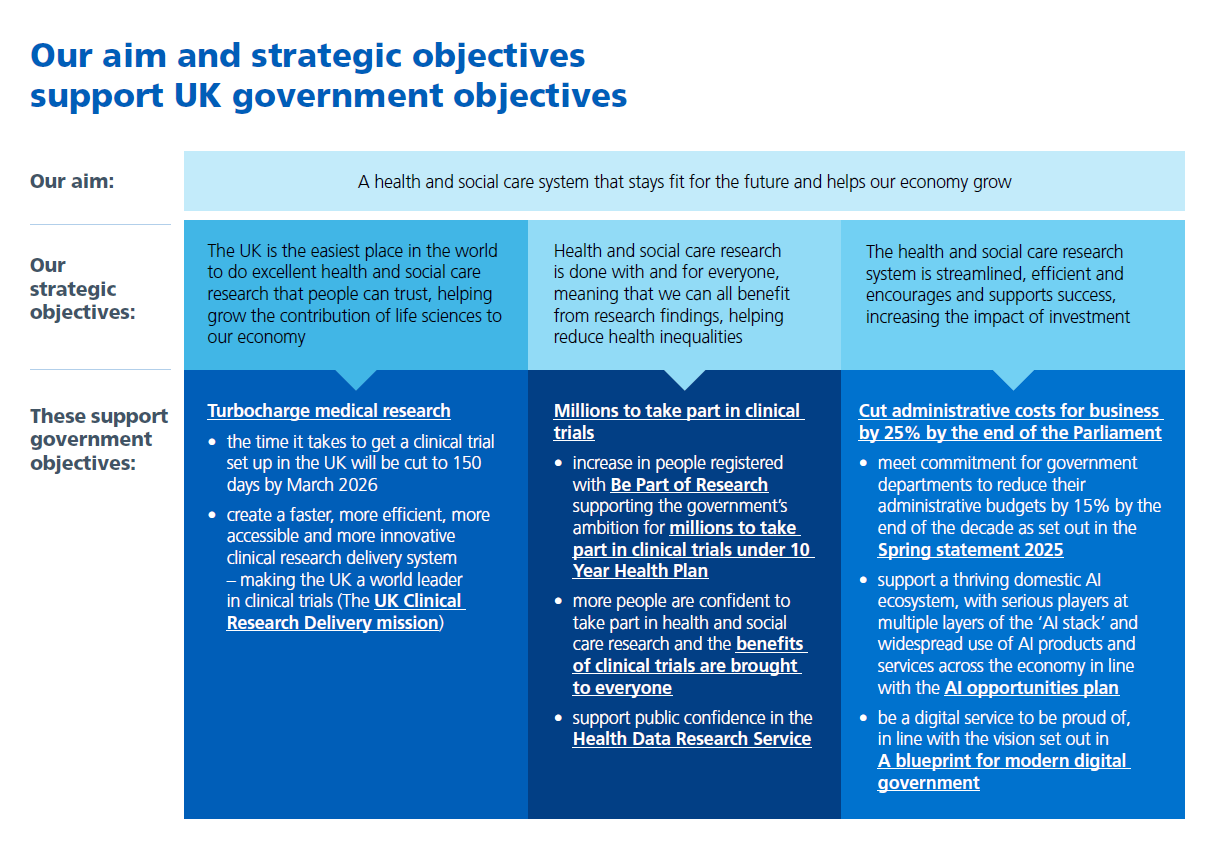
Our strategic aim for the next three years is to support these two missions, working towards a health and social care system that stays fit for the future and helps our economy grow.
Our objectives
We have identified three strategic objectives that our work is focused on helping to achieve over the next three years to support this aim:
- supporting UK growth in life sciences
- helping reduce health inequalities
- increasing the impact of money invested
Achieving our objectives
To do this, we know that we need to be a trusted and effective organisation that makes it simple and fast to do research well.
In our strategy we have set out how you will see us work in this way, with measurable outcomes that we will achieve over the next three years.
Simple
Outcomes that you will see
It is easier to do research across different settings in the UK, broadening access to research:
- there is a 10% increase in the percentage of people who tell us that it is easy to set up studies in the UK, including across NHS and non-NHS settings
- researchers have a positive experience finding out what they need to do and earning the approvals that they need with at least 75% of applicants rating our overall service highly
Research is more transparent with 100% of clinical trials registered and an increase in clinical trials uploading summary of results on a registry.
More research meaningfully involves the public to inform the way it is done shown by an increase in rates of public involvement in applications for review by a Research Ethics Committee.
There is greater representation in, and access to, research so that people from all backgrounds across the UK can take part in research relevant to them. This is shown by an increase in the proportion of researchers who use an Inclusion and Diversity Plan and are more confident to include a diverse group of people in their research.
Researchers can do research in new ways and using new technologies such as AI to improve care in a way that people can trust. We are clear how we support researchers to use new technologies and data to plan and do research, and to research the use of new approaches and technologies in care, in line with the AI opportunities plan.
Fast
Outcomes that you will see
It is faster and easier to use our new digital services to plan, approve, set up, manage and complete research in the UK. We have reduced the burden on users by improving end-to-end flow and replacing manual processes. We will deliver three core platform services by 2027.
Setting up research is more efficient with simple steps and no duplication. The burden, time and cost to do research has been reduced by standardising processes and reducing duplication in the set up of research studies, with a 20% increase in the percentage of people who tell us that the process of study set up is efficient.
We provide approvals in time to support the set up of clinical trials in 150 days:
- 100% of approvals of clinical trials are completed within a maximum of 60 days, with a median timeline below 40 days. This will support the ambitious target for clinical trial set up in the UK to be cut to 150 days and further in the longer term. We will continue to deliver faster timelines where appropriate
- you can get a decision on support for the use of confidential patient information without consent in England and Wales from the Confidentiality Advisory Group within 60 days or where a precedent has been supported within 30 days
- all research applications will receive an HRA and HCRW Approval decision in less than 60 days
Trusted
Outcomes that you will see
People can understand, value and trust us and the way that we work:
- sentiment analysis shows an increase in awareness and trust in the HRA across all stakeholders that need us
- we meaningfully involve people in the HRA’s work with at least 80% telling us that they feel that they had shaped and informed the work they had been involved in
- an increasing diversity of perspectives inform our work. We have set objectives for where we want to increase representation in our work and are working to achieve this
Our assurances are understood, valued and trusted, not unnecessarily repeated to set up research with a 20% reduction in the percentage of people who tell us that there is duplication in study set up.
Our digital services are secure, keeping them available and resilient to cyber attacks and meeting government assurance standards.
People know when and how to raise questions and concerns with us and we are clear how we will act on them, with 100% of complaints completed in 25 days and no more than 5% resulting in a subsequent appeal to the Chief Executive.
Effective
Outcomes that you will see
We are playing our part in reducing government running costs:
- we play our part in meeting the commitment for government departments to reduce their administrative budgets by 15% by the end of the decade
- we measure and report on the impact of our work on the productivity and efficiency of the health and social care research system
- we partner and collaborate and share services where this will help us deliver our mission and reduce costs
We are using AI well. We are using AI and digital technologies well and finding ways to use them more to improve the way we work and our services in a way that people can trust.
We have safe, scalable and secure digital infrastructure and the internal capability to expand, innovate and support future regulatory change.
Our staff have a high level of engagement. We maintain and grow our high level of staff engagement to 80% and maintain our strive measure at 85%.
Our community of volunteers and public contributors have a good experience with us and can see the difference they make, maintaining satisfaction at or above 80%.
The scale of change that we will make

You will see us transform, change and maintain and continuously improve services across all the areas that are set out in our strategy.
There are four areas where you will see the biggest change. These are where we can make impactful change fastest to support government objectives.
These are:
- we are part of modern, digital government. We will build our digital foundations as part of the vision for modern, digital government
- we are efficient and take action to increase productivity. We will embed productivity into everything we do, guiding our decision-making
- we give approvals and assurances, working in partnership UK-wide to provide a world-class regulatory system. We are focused on reducing the burden, time and cost to do research with and for everyone
- anyone can understand what we do and why it matters to them and be confident in our decisions. We will build the profile that we need to achieve our objectives well
The scale of change that we plan to make, including where we plan to change or maintain the way that we work, is indicated by the outcomes that we have set.
We work in partnership as part of the health and social care research system. This means that we will lead some change, but in other areas our activities and the changes that we make will support or align with work that is being led elsewhere.
Our level of focus and ambition is determined by where we can best support our strategic objectives, which align with government objectives, not where we lead, support or align with the wider system.
Over the next three sections we show the areas where we will transform, change or maintain and continuously improve our activities and services. In these areas we show what outcomes you can expect to see.
We have also identified where we will take the lead, support our stakeholders or align with other work already underway.
Transform
The four areas where we will lead and make bold, system-wide change.
We will rethink how we work, how we use our expertise and how we organise ourselves to deliver at scale and pace.
You will see us shaping a simpler, faster, more effective and trusted research system.
Lead
- building our digital foundations to be part of modern, digital government
- ensuring that anyone can understand what we do and why it matters to them and be confident in our decisions
Support
- reducing the burden, time and cost to do research with and for everyone to support the government target for the time it takes to get a clinical trial set up in the UK to be cut to 150 days by March 2026
- embedding efficiency and productivity into everything we do, guiding our decision-making
Change
The five areas where you will see us make meaningful and significant improvements.
We will redesign processes, update policies and introduce better tools and ways of working.
You will see us working with partners to embed more consistent, effective and user-centred approaches to regulate and approve research and show you the difference we are making.
Lead
- taking a proportionate approach to regulation without reducing standards
- encouraging and facilitating greater research transparency
- making decisions in a way that people can have confidence in
Support
- encouraging greater representation in, and access to, research
- encouraging and facilitating the use of AI and data in research and research into their use in care in a way that people can trust
Maintain and continuously improve
The nine areas where you will see us sustain our activities and services, building and embedding a culture of ongoing, incremental improvement.
We will refine existing systems, processes and practices to improve reliability, efficiency and user experience.
You will see us making steady improvements and helping others improve without disrupting essential activities and services or cutting corners.
Lead
- ensuring that our people can be their best
- giving approvals and assurances and helping co-ordinate a UK-wide regulatory system
Support
- encouraging and facilitating researchers to meaningfully involve the people their research is for and about
- encouraging and facilitating new ways to do research with public trust
Align
- encouraging and facilitating public health research
- encouraging and facilitating adult social care research
- encouraging and facilitating sustainability in research
- encouraging and facilitating international collaboration
- demonstrating the attractiveness of the UK as a destination for clinical research
How you will experience the change

Our work over the next three years will make some immediate contributions to the UK’s health and growth missions.
We will also make changes that will realise greater contributions in the future. For example, we are building digital foundations that will turbocharge our ability to make it easier to do research that people can trust as they come online. They will help us to standardise the ways that we measure and collect data, meaning that we can better understand the impact that our work is having and use these insights to inform decisions to make the greatest difference going forward.
Making change well is hard and progress will not be constant, so we expect outcomes to fluctuate up and down over the next three years. We will work within defined tolerances, aiming to achieve the outcomes that we have set in three years.
However, the world around us is always changing and what is needed to make it easier to do research that people can trust may change too.
Our focus on outcomes means that we can change the way that we work to achieve them, informed by what works and responding to external changes. This also gives us the freedom and flexibility to take opportunities presented by new technology such as AI, in line with the UK Government’s AI Opportunities Plan.
What changes you will see in each of the priority areas we have identified
Over the next three years you will see changes that broadly come under one of the following categories:
- we give approvals and assurances, working in partnership UK-wide to provide a world-class regulatory system
- we are part of modern, digital government
- anyone can understand what we do and why it matters to them and be confident in our decisions
- we set shared expectations for how to do research and encourage compliance with these
- our people can be their best
- we are efficient and take action to increase productivity
In each of the categories you will see an overview of the changes we will make and how this supports the government's ambitions.
Get in touch
If you have any questions about our strategy please email communications@hra.nhs.uk
View our previous strategies
Take a look at our previous strategies: