Contents
Transparency - leading the way to better health and social care research
Our commitment to drive research transparency
Research Transparency Annual Report 2021 - accessible version
Transparency - leading the way to better health and social care research
At the start of the COVID-19 pandemic it became clear that research was going to be the route to learn how to prevent, diagnose and treat the novel coronavirus. New studies were funded, reviewed and set up in record time. It was a heroic effort from the whole of the research community, and from the many members of the public and patients who contributed as participants. The success and impact of these studies on all of our lives was amplified by a commitment to collaborating and sharing information openly and transparently across the health and social care research system.
Sharing findings from health and social care research helps to identify better care and treatments and find the best ways for everyone to stay healthy and well. For research and care professionals, it avoids duplication of effort, helps to improve the quality of research and allows health and care professionals to embed research into everyday practice.
By making research findings public in a meaningful and timely way we acknowledge the commitment and value of the people who give their time and energy to take part. This openness also builds trust and accountability with the public, who we rely on to support efforts and participate in research in the future.
The Make it Public strategy is important for the whole of the health and social care community. It has set out what is needed to drive progress in research transparency. The Department of Health and Social Care gave its commitment to the Make it Public strategy when it was published in 2020 and will continue to support the HRA to make a real difference to transparency in research.

Professor Lucy Chappell FMedSci, Chief Scientific Adviser for the Department of Health and Social Care
Our commitment to drive research transparency
Health research is thriving across the UK. World-leading studies and trials are led here, working to tackle illnesses and disease, and to improve people’s health and wellbeing.
Robust and proportionate regulation is crucial to the success of these projects, and the HRA is committed to working with partners to deliver the commitments set out in Saving and Improving Lives, the government’s vision for the future of UK clinical research.
Underpinning these commitments is our unwavering commitment to transparency, the cornerstone of high-quality health and social care research.
Your feedback, given as part of our consultation, helped shape the Make it Public strategy that we have today. We commit to change, support and encourage the research sector to always be transparent and open, and deliver the benefits this offers everyone.
The strategy was launched in July 2020, and although we had to adjust our timeline because of the ongoing pandemic, we have been making great strides to implement and champion transparency and to make it the norm across the health and social care sector.
There’s more information about the changes we’ve made and the benefits these will bring in the following pages. In future years we’ll build on our new ways of working, using them to audit and report on how well the research community is fulfilling its requirements.
Dr Matt Westmore, Chief Executive, Health Research Authority

#MakeItPublic Strategy
Being transparent about what research is happening, and what the findings are, is essential for patients, public, and professionals.

Being transparent about what research is happening, and what the findings are, is essential for patients, public, and professionals. It avoids duplication of effort, enables findings to be used to develop new and better treatments for patients and service users and helps us to stay well. Transparency is key to the thriving health and social care research environment in the UK. More than one million people took part in COVID-19 studies in the UK in the first year of the current pandemic.
Partners across the research sector, including the NHS, regulators, medical research charities, life sciences industry, the UK government and devolved administrations, are now working together to learn from the emergency response and create the strongest possible research environment. Research transparency is central to this.
The HRA developed #MakeItPublic to bring about a step change in research transparency across the UK health and social care research sector. Responding to recommendations for change made by the House of Commons Science and Technology Committee, we set out to make transparency the norm.
The strategy set out our commitment to change under a simple vision: that trusted information from health and social care research studies is publicly available for the benefit of all. We have been working over the last year to make that happen.
We are making changes to:
Registration: making it public that a study has started
To avoid duplication and waste, information about research projects should be made publicly available before they start. In the case of clinical trials, this means before the first patient is recruited.
In early 2022, we’re starting a new automated registration service for clinical trials. There is more on this later in the report.
Reporting Results: making it public what the study has found
It is important that the results of individual research studies are shared publicly and made accessible, whether those findings are positive or negative. For clinical trials, as a minimum, the record in the registry should be kept updated
as the study progresses, including adding a summary of the results.
We have made big steps forward in this area: updating guidance and changing the way we collect information at the end of the study to check whether research teams are being transparent. Next to come is what we do with this information. Find out more in the later section.
Informing participants: letting those who took part know what the study has found
Information about the findings of the research should be available, in a suitable format and timely manner, to those who took part in it. Giving participants information about the findings of a research study is a key aspect of research transparency. It respects participants and acknowledges their contribution. We’ve begun by asking researchers how they plan to inform participants about their findings and checking that they have done that after the study has finished.
Sharing study data and tissue: enabling further research
Sharing tissue and data (with the right protections in place) is important to further scientific knowledge and maximise the time and efforts given by participants.
We have already made great progress towards the first three points, and our plan sets out what else we will have achieved by March 2022.
This strategy was developed with the help of the Research Transparency Strategy Group and we are now pushing it forward in partnership with the Make it Public Campaign Group.
Professor David Crossman, Chief Scientist (Health), Scottish Government‘We know the importance and value of health and social care research; but recognise greater transparency is needed - not only in the research taking place, but the findings of that research. We are proud to have been part of the development of the Make it Public strategy with HRA and partners across the sector. It is an important step forward and will benefit the patients and public who participate in vital research; the teams who set up and deliver research and the many partners who use that research to make decisions. We will continue to support the ambitions of the strategy - ultimately maximising the benefits of research for all.’
Alex Newberry, Health and Care Research Wales‘The whole research community understands the need for transparency, but it’s not always clear how to do that without a large increase in burden. That’s why it’s important that the HRA is taking a leading role in guiding this work. We at Health and Care Research Wales are proud to be able be a part of this important work by ensuring delivery of the Make it Public strategy aims are incorporated into our policy development and funding call requirements.’
Jennifer Harris, PhD, Head of Research Policy, The Association of the British Pharmaceutical Industry‘The Make it Public strategy sets out a vision for health and social care research findings to be publicly available for the benefit of all. ABPI fully supports this vision and will continue to work collaboratively to implement this strategy in order to enhance opportunities for research involvement and improve research transparency.’
The four pillars of transparency – progress report
Registration

Making studies visible before recruitment begins reduces duplication and research waste and protects participants from unnecessary research. Clinical trials must be added to a publicly accessible register before the first participant is recruited, or no later than six weeks afterwards, but this doesn’t always happen. Over the last three years our own audits have shown an almost 9% increase in compliance, but still more than one in ten clinical trials are not registered as they should be.
Registration is good practice for all health and social care research studies – not just clinical trials. It’s fundamental to research being transparent.
Sponsors are responsible for registering studies, but because this is so important, we’ve committed to make sure it happens. We commissioned an independent agency to assess the different options and we’ve partnered with ISRCTN registry, a UK-based registry recognised by the World Health Organization (WHO), to develop a new service.
The service will link HRA and registry systems to automatically register all UK clinical trials once they have received a favourable ethics opinion. This removes the burden from trial managers and will mean that all UK clinical trials will be registered in the same place. There will be no charge for the service which is part of our commitment to making transparency easy for health and social care researchers.
The new registration service is being piloted ahead of a full launch with the first phase, for clinical trials of medicines, to go live in 2022.
Marc Taylor, Chair, ISRCTN Registry‘Trial registration sets the foundation for clinical research transparency and transparency is at the heart of what we do at ISRCTN as a primary clinical trials registry in the WHO system. We’re proud to collaborate with the HRA in this work to improve transparency across the research community and to see the impact of the steps that have already been taken.’
Dr Aoife Regan (PhD), Head of ECMC Programme Office, Experimental Cancer Medicine Centre (ECMC)‘It’s fantastic to see the Health Research Authority leading the charge on research transparency, working collaboratively with partners and with public input throughout.
We look forward to working together with the HRA and the research community to make lasting change in this area.’
Reporting Results

When studies have finished, it’s crucial that the results are reported. Reporting results for all studies, whatever the outcome, prevents duplication and research waste. It also ensures that results can be used to inform policy or care that further improves health and wellbeing. High quality research, even where outcomes are negative or inconclusive, always reports results so that they are publicly accessible.
Publishing results in a peer-reviewed journal is important but it isn’t always achievable or the findings then accessible to the public. Results should be made available, at least in summary form, in a place where they can be seen and the findings should be summarised in a way that can be understood by the public.
We have made significant changes to increase results reporting for all health and social care studies.
First, we strengthened and improved our guidance to make it clearer for researchers to know what is required. When they apply to us for permission to start their research we’ve made sure that our expectations about reporting results are clear from the very beginning.
Then we changed the way that we collect information at the end of a study. For the first time there is a standard set of information that we expect to be reported to us when research has completed, including a confirmation that the results have been published. This is another way in which we’re pushing to make transparency the norm.
We’re now working with the Medicines and Healthcare products Regulatory Agency on how we can share information about the summary of results we collect in our final reports. As both organisations ask about summary of results, we want to reduce reporting burdens for sponsors and share data and knowledge in order to further the transparency agenda.
Next, we’ll use our records to follow up with studies that have not yet submitted their end of study information to us, and in the future we’ll use this to make it clear when studies have unreasonably failed to report on time.
Della Dolapo Ogunleye, Patient Contributor‘I strongly believe that diversity in research and service design is vital to ensuring treatment and care is tailored to everyone’s needs. To improve diversity in design and participation you must first foster trust, this is particularly important when trying to reach under represented communities. Increased transparency and sign posting to clear information is a key way to build that trust in research among patients and the public, which is why Make it Public is vital for us all.’
Informing participants

Finding out about the outcome of research is important for patients, service users and healthy volunteers who take part in research. It respects the contribution that they made to the study and means they are more likely to take part in future health and social care research. If findings are not shared in a meaningful and timely way, it diminishes the commitment and value of the people who have given their time and energy to take part.
To encourage researchers to plan early for informing volunteers, we’ve changed the question we ask applicants from whether they will share study results with participants to how and when they will share them. We’re also developing new guidance on how to inform participants about study findings which takes into account types of research which might make this more of a challenge.
We now ask for plain language summary of the study findings to be submitted as part of the final report. By publishing these on our website, we will help to make the results accessible to other people interested in the study, including professionals, commissioners, policy makers, and funders to help make informed decisions about their work. This will also support wider public engagement with, and promotion of research.
Researchers told us that they don’t always know how to write a summary which explains their research to everyone, so this year to support sponsors and researchers in communicating results, we have developed an e-learning module to help them produce more consistent plain language summaries of their research.
Lynn Laidlaw, Public Contributor‘Expecting researchers to inform participants about the results of the research they took part in, rather than asking them will they do so, is a large, symbolic shift that the Health Research Authority is making. It sets an expectation to everyone that informing research participants of the results is the normal, and right, thing to do.'
Jo Taylor, Founder, After Breast Cancer Diagnosis‘I am pleased to see not only the Health Research Authority, but also the wider research community’s commitment to improving transparency. Communicating with participants is quite simply the right way to do research, it acknowledges the commitment of those participants and lets us know that our effort has been meaningful. To then make those results public, really maximises our involvement. It means all that we’ve given could then go on to guide further research.’
Sharing study data and tissue
Our Make it Public strategy focusses on the first three pillars of research transparency, which are priorities for the HRA.
The fourth pillar, sharing study data and tissue, is also crucial to enable further research and this is something that we will be promoting through the Make it Public Campaign Group.
Make it Public is a strategy for the whole research community. There are other organisations that are well placed to progress the fourth pillar and are already doing great work in this area.
Here are just a few of those:
Platforms supporting research transparency
Wellcome Open Research helps the organisation’s grant holders to rapidly publish all outputs from their research – everything from articles and datasets to case reports, protocols, and null and negative results.
The system, developed by F1000Research, was launched in 2016 and ensures funded research can be shared in an open and transparent way.
It’s intended to drive scientific progress and help grant holders to try out new approaches.
The Association of Medical Research Charities’ Open Research is a publishing platform that helps researchers funded by medical research charities rapidly publish any results they think are worth sharing.
To support transparency, reproducibility and impact, researchers can share products from their research from study protocols to articles, systematic reviews and open letters.
As the peer review process happens after publication, the article is available within days of submission. Researchers are also able to publish supporting data enabling reanalyses, replication and reuse.
The National Institute for Health Research’s journals library is a full archive of funded research which means all NIHR studies are fully transparent and available in the public domain.
Each study has a thread which aims to tell the full story of the research, from inception to final publication and dissemination of study results. This includes a published final report.
The journals library also supports researchers to publish findings in external academic journals, making sure to minimise duplication.
The NIHR has also enabled the publication of reports that are of wider public interest, even though this work may have originally been funded through another public body. The organisation is committed to support the publication and transparency of key research that will support the discovery and transparency of important findings.
Dr Phil Quinlan, University of Nottingham‘Sharing of study tissue and data is an essential pillar of transparency. With the right safeguards in place it can give researchers access to a wide range of diverse data. We owe it to participants who take part in research for their involvement to be leveraged to maximise scientific knowledge. This is why I am pleased to see the importance being placed on this area alongside the wider transparency strategy.’

The next phase of Make it Public
Making transparency easy
Our new transparency roadmap means that researchers can see at a glance where they need to take action. The map traces a study journey from the design and planning stage to completion of research.
An interactive version on the HRA website allows sponsors and study teams to easily access supporting information such as guidance and advice by clicking on the transparency touchpoints.
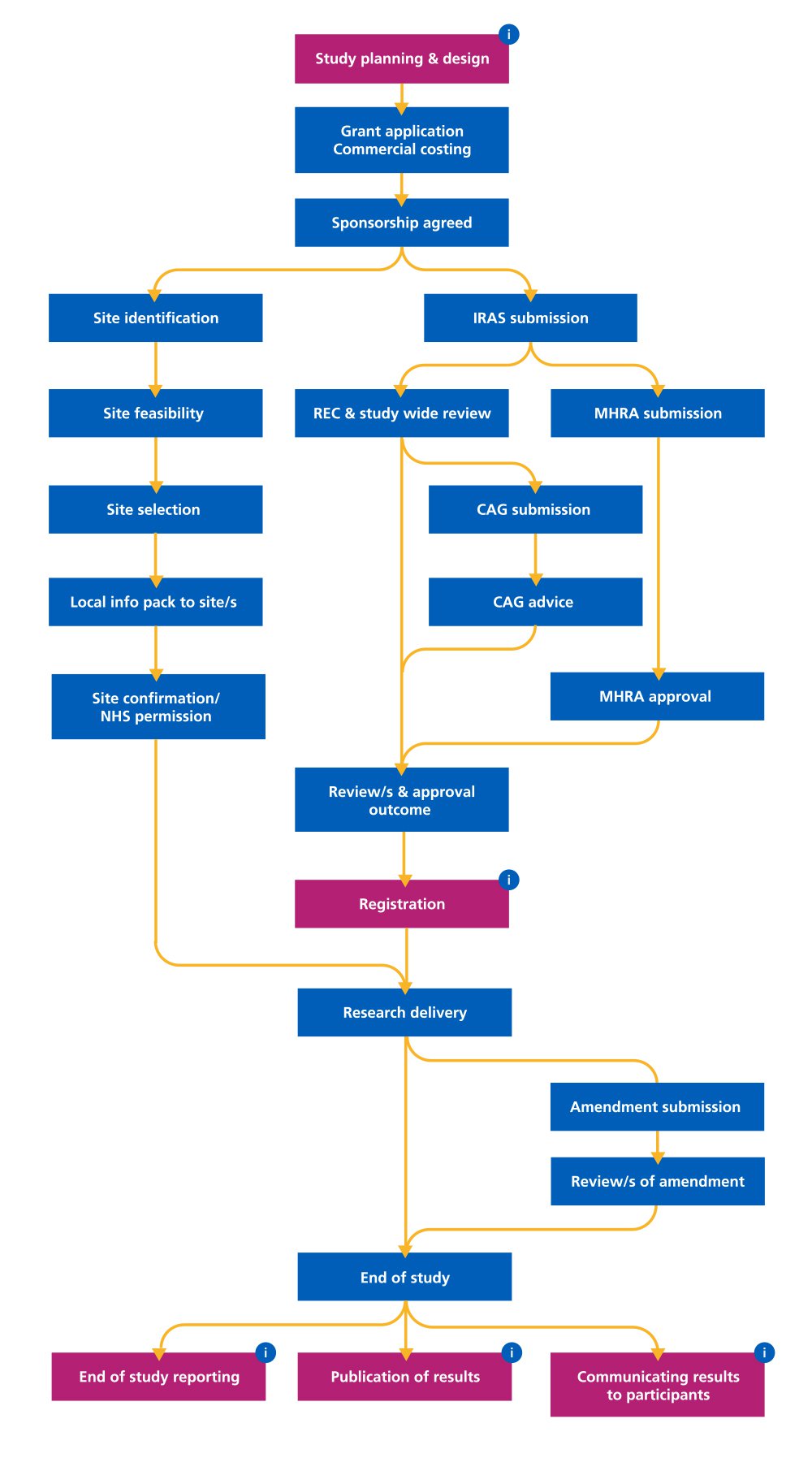
See our accessible interactive view of the journey an average clinical study takes.
Angela Williams, Head of Research & Development, North Central London Research Network (NoCLOR)‘At NoCLOR we had the opportunity to influence how the research transparency road map was shaped at a local level, and through our involvement we felt we could make a difference to the wider transparency landscape. Making research more accessible to the public and finding the evidence to improve patient care is at the heart of the Make it Public strategy and will not only have an impact on the public but the wider research community’.
Making transparency happen
The Make it Public Campaign Group in action
We brought together our Make it Public Campaign Group at the beginning of 2021, and asked them to work with us to support and encourage researchers and research sponsors to be transparent, and to champion the new ways of working to make sure this is always the case in health and social care research.
The Campaign Group takes a collaborative shared learning approach and explores ways to increase awareness and confidence in research transparency, driving the development and implementation of the transparency strategy across the health and care sector. It is co-chaired by Matt Westmore, Chief Executive of the HRA, and Derek Stewart, a patient representative.
Matt‘The Make it Public Strategy is not just a strategy for the HRA, it’s a strategy for the whole research community. It was developed with the help of people from devolved nations, industries, funders, charities, academics, researchers, and patient representatives so it’s only natural that we all continue to work together to make it happen in practice. Every organisation represented on the campaign group is committed to making transparency easy and making it the norm. With so many committed and passionate people all pulling towards the same goal I’m sure we can make lasting change for the benefit of all.’

Derek‘Public and patient involvement is essential to clinical research but for this to happen the research studies have to be transparent and easily accessible for the public. It’s been a great pleasure working with the campaign group to help shape, influence and transform how we as a nation become transparent with our research.’
Members of the group include Health Research Authority and colleagues from the Devolved Administrations, Association of the British Pharmaceutical Industry, Wellcome Trust, National Institute for Health Research, Medical Research Council, TranspariMED, Cancer Research UK, World Health Organization, Ethical Medicines Industry Group, Association of Medical Research Charities, and patient representatives.
The year ahead
Working alongside our Campaign Group and partners in the research landscape, we’ll carry on working to fulfil the commitments in the Make it Public strategy. We are determined to bring about lasting, meaningful change in research transparency practice.
Although our timelines have been disrupted by the COVID-19 pandemic, the changes implemented in 2020 are already taking effect. With changes to reporting at the end of studies, we will be able in our next annual report to report on transparency performance across the sector. As always, you can follow our progress at www.hra.nhs.uk
| Pillar | Activity | Delivery date |
| Registration | Introduce automatic registration of UK clinical trials on ISRCTN | January 2022 |
| Reporting results | Design and implement automated reminders and electronic submission of final reports for all research | January 2022 onwards |
| Reporting results | Establish a stakeholder forum to help us better align final reporting requirements for regulators and key funders | March 2022 |
| Reporting results | Develop a policy for how we will assess performance against research transparency requirements | March 2022 |
| Reporting results | Launch a new and expanded Research Summaries tool on our website |
December 2022 |
If you have comments or questions on anything in the report please contact Research.Transparency@hra.nhs.uk