From 28 April 2026, changes to all approved studies will no longer be called 'amendments'. Instead, they'll be referred to as 'modifications'.
To familiarise yourself with the different type of modifications, we recommend you first read the update to 'amendment' terminology for non-CTIMPs guidance which details the changes.
We're designing and building a new digital service, Plan and Manage Health and Care Research, that will replace existing systems for planning, approving, setting up, managing and completing research. We introduced the new service to a small number of users in December 2025. We're using the term modifications for all study types in the new service.
The following sections outline the approvals process and timeframes from 28 April 2026 for modification requests, which will be reviewed by a Research Ethics Committee (REC).
The following guidance does not set out the processes and timelines for other reviews a modification may need, such as HRA and Health and Care Research Wales (HCRW) Approval.
Modification timeframe and process
Categorising and submitting modifications
From 28 April 2026, if you need to submit a modification, the process for doing so will follow the current process for submitting modifications. You will still need to complete the Amendment Tool (which will then be the ‘Modification Tool’), capturing the details of the trial and the proposed changes.
The tool will then categorise the modification based on the information you input, give you guidance on which regulatory bodies (for example the REC, HRA) may need to review the modification and how you should submit it.
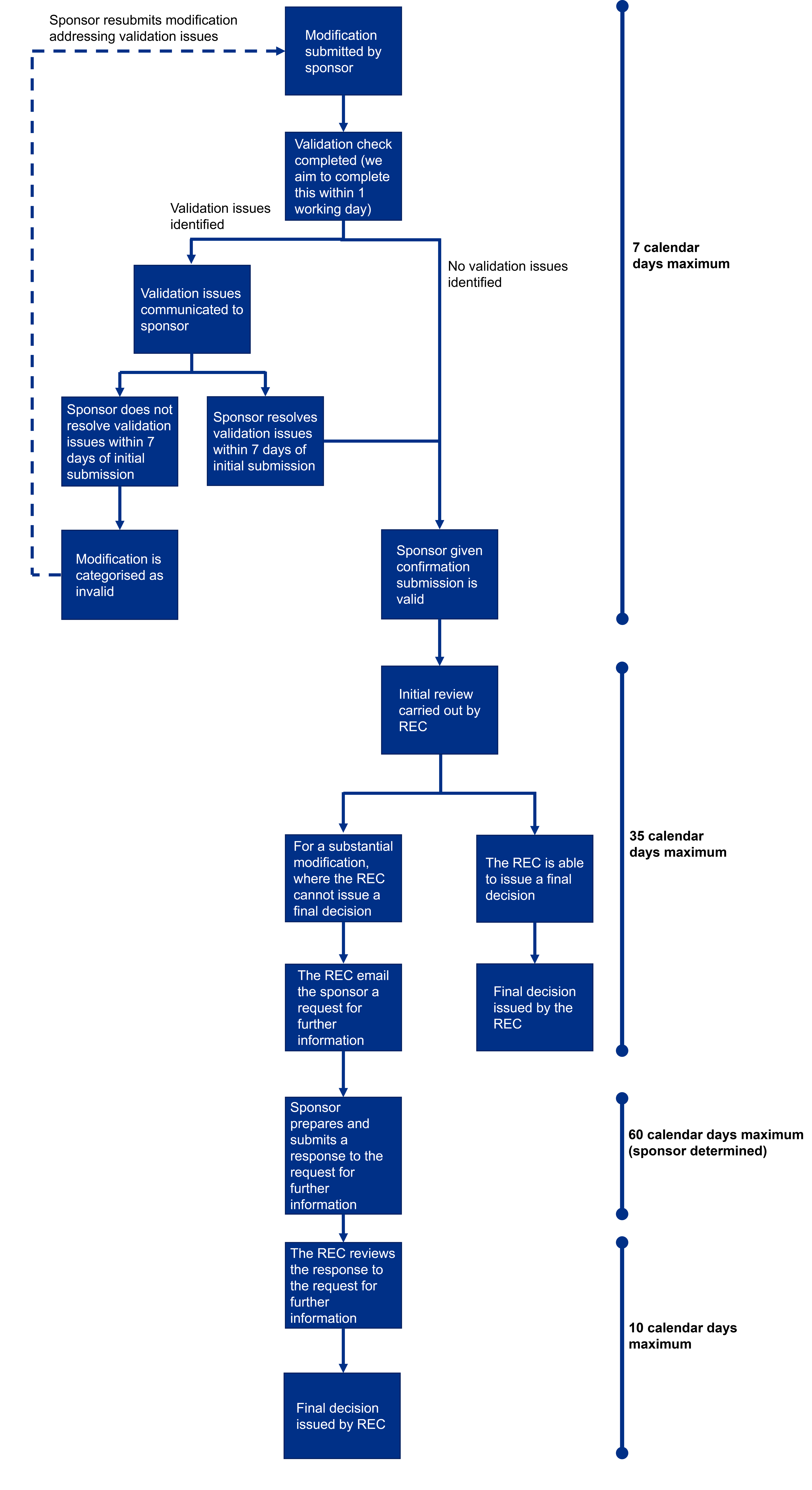
Substantial modification request validation
When a sponsor submits a substantial modification request, the REC will check to make sure it’s valid. We’ll aim to communicate the outcome of the validation check within 1 working day of it being submitted.
If the REC identifies any issues that prevent a substantial modification request from being considered valid, the sponsor will be asked to address these issues within 7 calendar days of when they submitted the substantial modification request.
If they cannot address these issues within 7 days, the REC will categorise the substantial modification request as invalid. This means the sponsor will need to resubmit the substantial modification request, making sure they address the validation issues raised by the REC.
In all cases a substantial modification request will either be confirmed as valid or invalid within 7 calendar days of the modification being submitted. The sponsor will be notified via email to confirm if the substantial modification request is valid or invalid.
Once a substantial modification request is confirmed as valid, it will be reviewed and an outcome will be issued within 35 calendar days.
Initial review outcomes
There are 4 possible outcomes from the initial REC review of a substantial modification request. These are:
- favourable opinion
- favourable opinion subject to conditions
- unable to issue a favourable opinion and requests further information
- unfavourable opinion
Requests for further information
The revised process will allow the REC to request further information when considering substantial modification requests.
This means that if the REC identifies issues that prevent a substantial modification request from being approved, the sponsor will be informed of these issues and will be able to respond before a final decision or opinion is issued. Requests for further information for substantial modifications will only be issued in a minority of cases, where it’s not possible for the REC to give a favourable opinion or a favourable opinion with conditions.
If a request for further information is required, it will be sent within 35 calendar days of the substantial modification request being confirmed as valid. If a sponsor receives a request for further information, they’ll have a maximum of 60 calendar days to respond. A sponsor can respond at any point within the 60 day timeframe. If they do not respond within 60 days, the substantial modification request will not be approved and will be given an unfavourable opinion by the REC.
If the sponsor needs longer to prepare a response to the request for further information, they can request an extension from the REC.
They will need to explain in their request why they need an extension and when they expect to respond.
Modified amendments
The introduction of the request for information process removes the option to submit a modified amendment. The process before 28 April 2026 allowed submission of a modified amendment following an unfavourable opinion.
However, from 28 April 2026 the option to provide further detail to the REC will be provided before a final decision is given.
Modified amendments must not be submitted.
REC reviewing a response to a request for further information
Once the sponsor submits a response to a request for further information, the REC will decide the outcome within a maximum of 10 calendar days. This will be communicated via email.
There are 3 possible outcomes from the REC after they review a response to a request for further information. These are:
- favourable opinion
- favourable opinion subject to conditions
- unfavourable opinion
If a substantial modification request is given an unfavourable opinion, the sponsor can appeal by emailing us at appeals@hra.nhs.uk within 28 calendar days of receiving the outcome. The sponsor will need to outline why they disagree with the outcome issued for the modification when they submit their appeal.
Clinical trials regulations guidance
For more information about the changes to CTIMPs, read our clinical trials regulations guidance.