The timelines set out in the Medicines for Human Use (Clinical Trials) (Amendment) Regulations 2025, which came into effect on 28 April 2026, also apply to Phase 1 healthy volunteer trials.
The initial application and modification section of this guidance explains the maximum time allowed for each stage of the approval process for all clinical trials of investigational medicinal products (CTIMPs), including Phase 1 healthy volunteer trials. We’ll meet these timeframes in all cases. We’ll also continue to process applications and modifications as quickly as possible.
We recognise that predictability and speed are particularly important for Phase 1 healthy volunteer trials that take place in the UK. We’ll continue to help make sure the approval process for these trials is predictable and efficient.
Fast-track research ethics review service
We’ll aim to provide the fast-track research ethics review service for all Phase 1 healthy volunteer studies. This will allow all Phase 1 healthy volunteer trials in the UK to benefit from a rapid ethical review process, reducing the time from application to outcome.
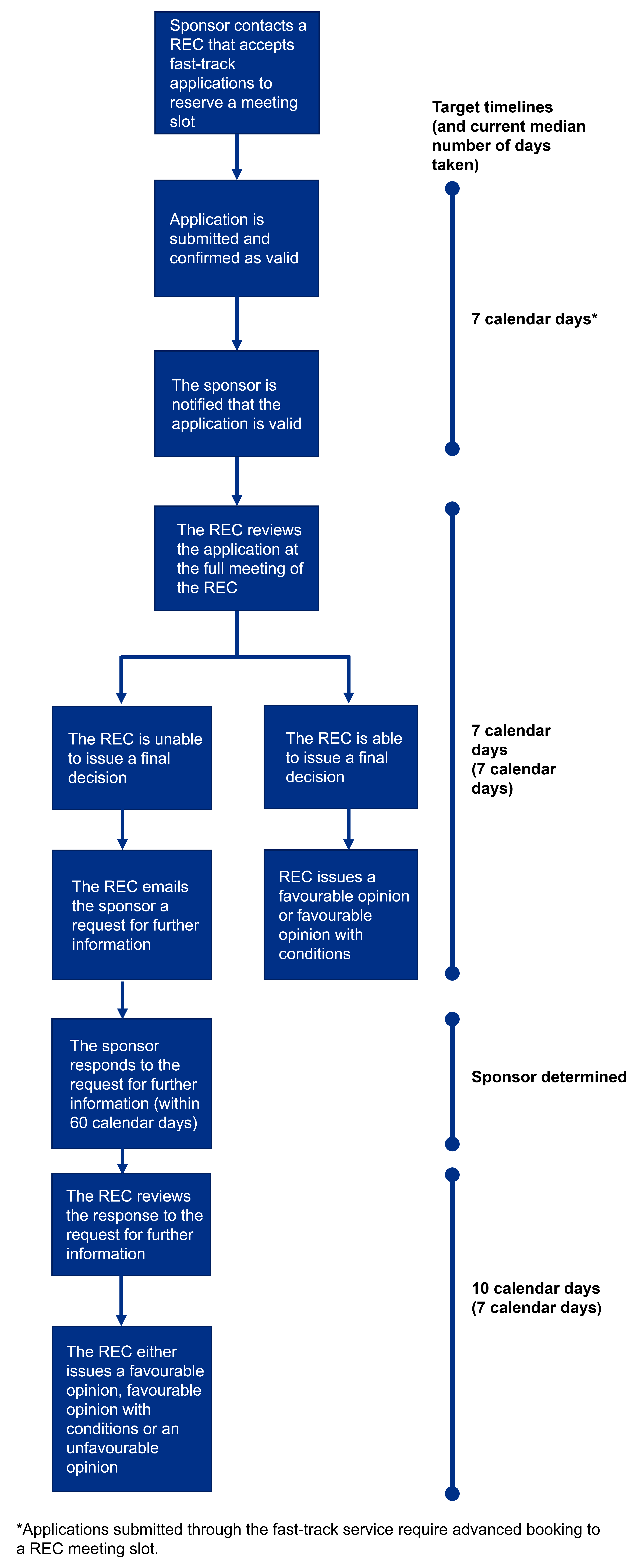
For all Phase 1 healthy volunteer studies submitted through the fast-track service, the assessment period begins 7 calendar days before the selected Research Ethics Committee (REC) meeting. We’ll aim to share the REC’s outcome based on the initial review within 7 calendar days after the REC meeting.
This means all Phase 1 healthy volunteer trials submitted through fast-track will receive the initial outcome from the REC within 14 calendar days from the start of the assessment period.
REC target timelines for fast-track REC applications
The following diagram outlines the REC target timeframes for all Phase 1 healthy volunteer applications submitted through the fast-track research ethics review service. Please note this diagram does not include the timeframe for Medicines and Healthcare products Regulatory Agency (MHRA) assessment of Phase 1 applications.
The target timelines we have for each part of the process are detailed on the right side of the diagram, in addition to the current average time taken for this to be completed for Phase 1 trial applications (based on performance data for fast-track applications reviewed by RECs between 1 April 2024 and 31 March 2025).
Target timelines for REC review of substantial modifications to Phase 1 trials
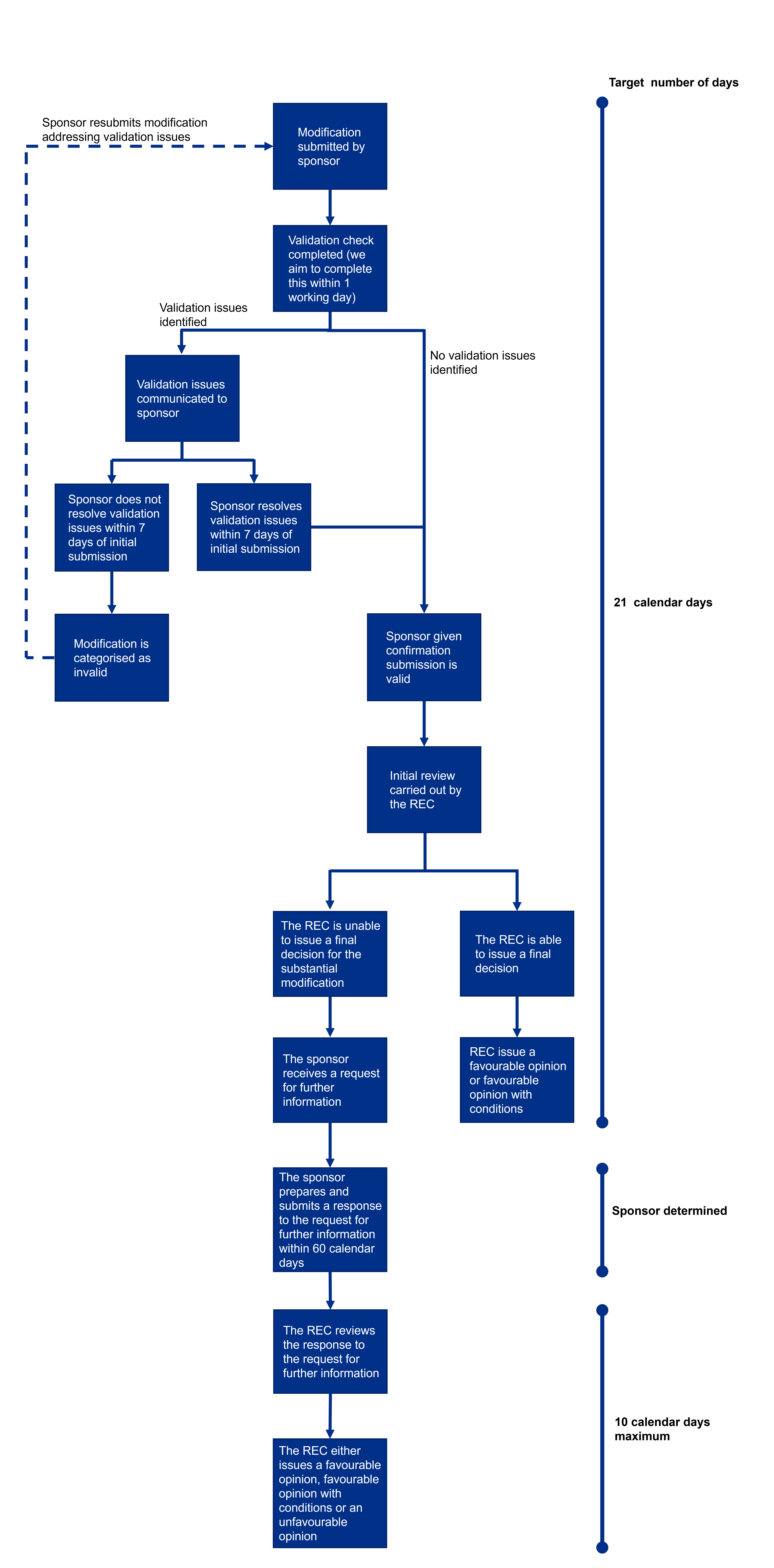
We’ll aim to issue the initial outcome from the REC for substantial modifications within 21 calendar days of receiving the substantial modification request. The criteria for how RECs review and assess substantial modifications have not changed.
While the 2025 regulations allow RECs to request further information for substantial modifications, these will only be sent in situations where the REC cannot otherwise give a favourable opinion or a favourable opinion with conditions.
If the REC sends a request for further information, they will issue a final decision within a maximum of 10 calendar days once they receive a complete response.
REC target timelines for modifications to Phase 1 healthy volunteer trials
The following diagram outlines the target timeframes that RECs will work towards for modifications to Phase 1 healthy volunteer trials. Please note this diagram does not include the timeframe for MHRA assessment of Phase 1 modifications.