Pharmacy Assurance is an integral part of study set-up in the NHS and HSC. We currently accept all Phase I-III clinical trials of investigational medicinal products (CTIMPs) when:
- the trial is taking place at multiple secondary care sites in the NHS and;
- the lead NHS R&D office is in England or Wales
It is expected that NHS sponsors in England and Wales submit their eligible studies through the process from 1 April 2020. The process will continue to be available for other sponsors with eligible studies.
Other study types will be accepted in England and Wales at a later date.
Please note that single site studies will not be accepted in England and Wales.
Information about applying for Pharmacy Assurance in Scotland and Northern Ireland is available on the IRAS website.
How does the process work?
Pharmacy Assurance is a pre-submission review, which takes place before e-submission to the REC. This is to ensure information is available to sites in good time to conduct their capacity and capability review. We recommend studies should be submitted for Pharmacy Assurance no later than three weeks prior to submission for REC review.
When a study is submitted for Pharmacy Assurance, we will check it has all the required documents. You can see what these are on the IRAS website.
All documents should be provided to the Technical Assurance team via email to pharmacy.assurance@hra.nhs.uk.
You will also need to tell us how you would like your study to be processed.
There are two management routes for Pharmacy Assurance:
Both options are available to studies meeting the roll-out criteria where the lead nation is England or Wales.
HRA-managed
This process is managed by the HRA on behalf of the applicant and involves a review fee payable to the reviewing trust; our review fee page has further information. The HRA-managed option is not available to single site studies.
Applicants submitting studies through the HRA-managed route have the choice to request up to three trusts or health boards for the HRA to approach to undertake the review. Alternatively, we will select HRA registered reviewers with the appropriate experience in the study field, such as paediatric oncology.
There is a process flow chart for the HRA-managed route below.
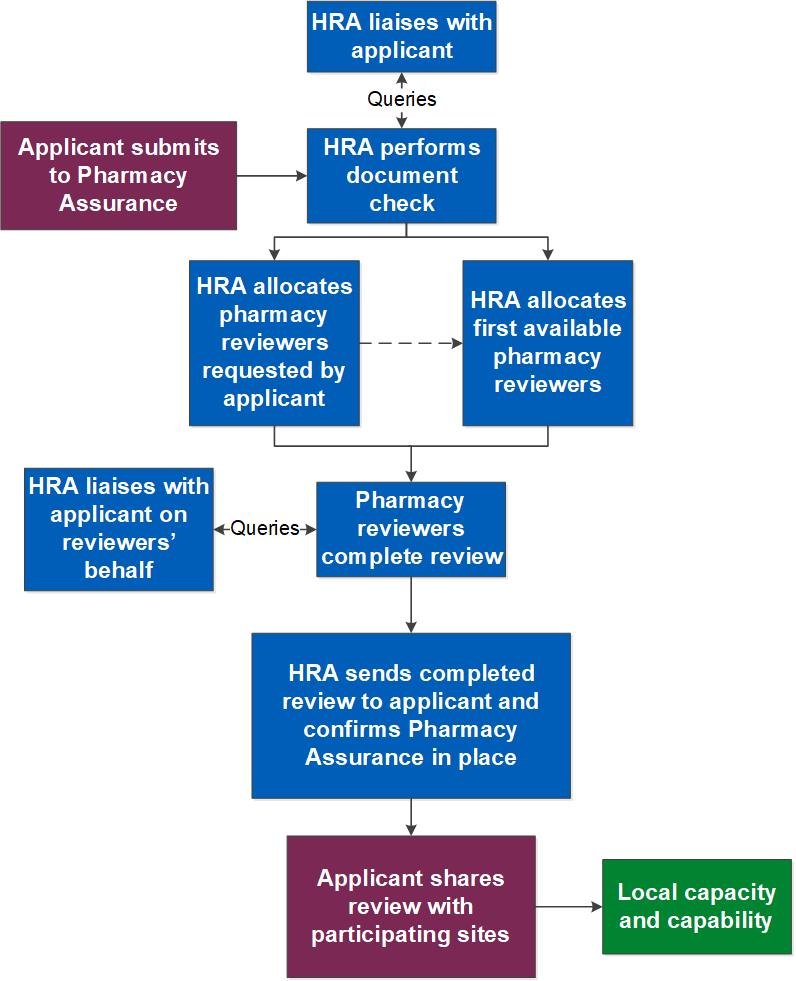
Self-managed
This option is available to non-commercial studies where the sponsor (or co-sponsor) is the same as the reviewing trust or health board.
Applicants submitting studies through the self-managed route will manage the selection of reviewers and the review process. Reviewers undertaking the review on behalf of the applicant should meet the following criteria:
- be registered with the HRA
- be based at the sponsoring trust/health board
- had input into the development of the study documents and set up of the study (in particular the sourcing, packaging, and labelling of IMP(s)).
You are expected to specify which reviewer(s) have been selected when registering your application for self-managed review. You will need to send your completed review to us for confirmation of Pharmacy Assurance. If you have reviewers you prefer to use at your trust/health board but who are not currently registered with us, please encourage them to register as reviewers with us. More information is available on our reviewer registration page.
There is a process flow chart for the self-managed process below.

What will the timelines for review be?
During the roll out phase we are testing a timeline for HRA-managed studies of 30 calendar days. As studies come in and are reviewed, we collect data about the actual time taken for Pharmacy Assurance and may adjust timelines in the future.
For current metrics please see our main Pharmacy Assurance page.
Feedback from both commercial and non-commercial sponsors has shown that the process makes site set up quicker and easier prior to the start of a study.
Still got questions?
Visit our FAQs page.