Findings from the online user satisfaction survey
The following graphs present quantitative data collected between October 2024 and September 2025 using the HRA online user satisfaction survey. This report does not include the free text qualitative comments - these are reported to the management teams in monthly reports.
For the last reporting period (April to September 2025) 286 respondents completed the survey. Response rates have slightly increased from the last reporting period, with 274 respondents completed the survey for the reporting period October 2024 to March 2025.
HRA Overall Service
Figure 1 - overall mean satisfaction scores for the period October 2024 to September 2025.
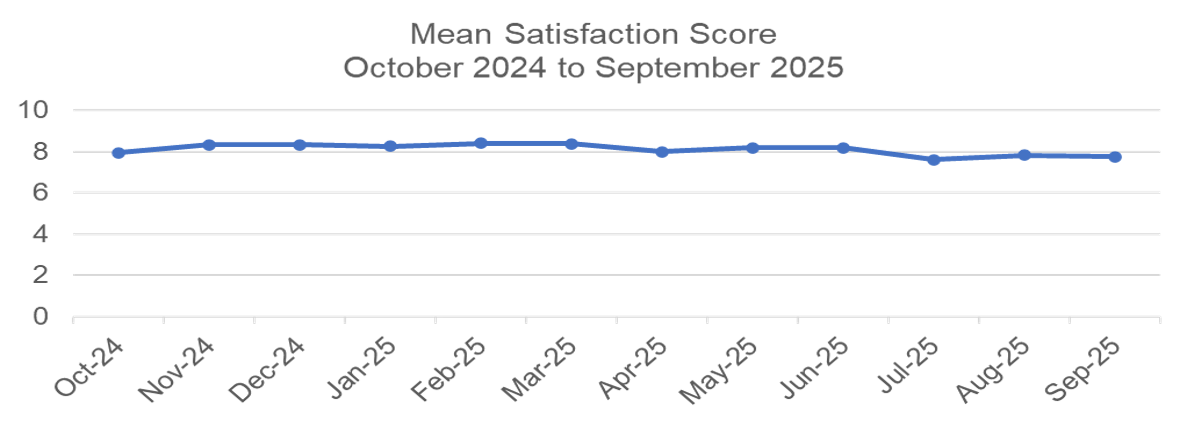
The graph maps the mean overall service satisfaction score for each month over the last year (October 2024 to September 2025). The chart details a relatively stable mean score - with the highest score in February and March 2025 (8.4). The level of satisfaction in September 2025 (7.8) was lower than at the beginning of the 12 month period in October 2024 (8.0). The lowest score during the reporting period was 7.6 in July 2025.
Download a csv file for graph data from figure 1.
Users' experiences of different aspects of HRA Services
Figure 2 - mean satisfaction scores broken down by category for October 2024 to September 2025.
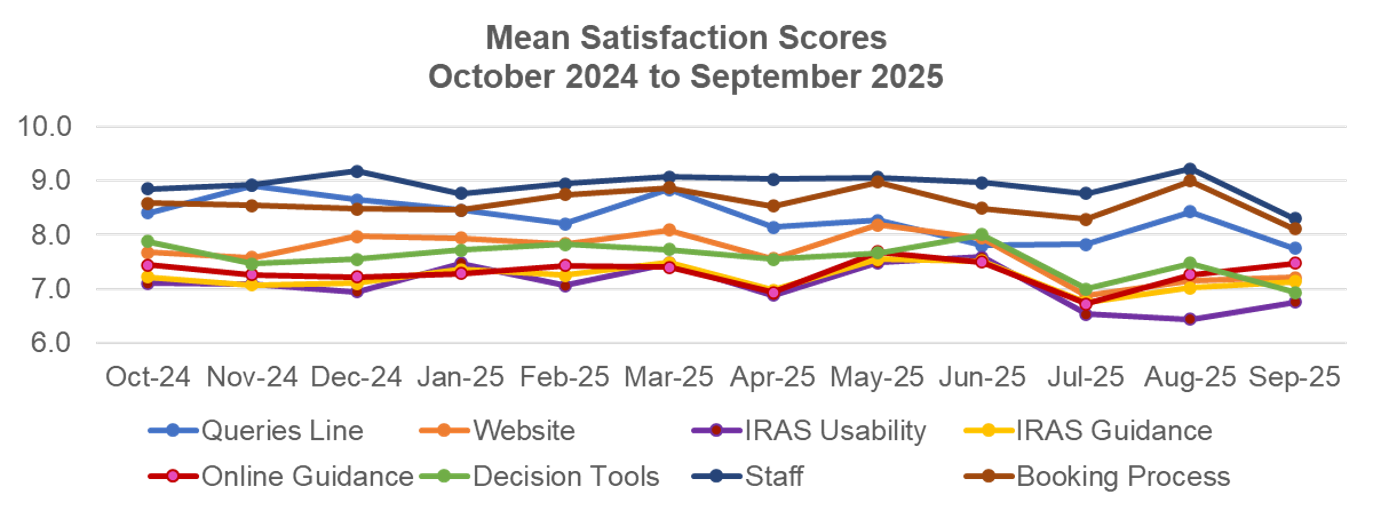
The graph details the mean scores for the following areas: queries line, HRA website, IRAS usability, IRAS guidance, online guidance, decision tools and staff, over a 12 month period from October 2024 to September 2025. The mean scores fluctuate during the 12 month period in all areas. The highest mean score was for Staff in December 2024 and August 2025 at 9.2. The lowest mean score was for IRAS Usability in August 2025 at 6.4.
Download a csv file for graph data from figure 2.
Review and action taken in respect of the issues raised in the user satisfaction feedback report for the period October 2024 to March 2025.
The following section outlines the review and action taken by the HRA functions in respect of the issues raised by applicants through user and stakeholder feedback.
HRA Approval
Aligned processes between NHS England and Confidentiality Advisory Group (CAG)
Meeting with NHS England to discuss how HRA and NHS England processes can be better aligned and where NHS England's Advisory Group for Data (AGD) can take assurance from regulatory reviews already undertaken by CAG/ethics.
Information around Mental Capacity Act in minutes not as clear as could be
Creation of a tool that makes the question set required for mental capacity act review clearer and more accessible for research ethics committee (REC) members and the REC team. This has been piloted with 6 RECS and proved successful. The next steps in discussion currently are rolling out across operational teams working with RECs in England and Wales, where the MCA applies and fine tuning the tool through feedback.
New Clinical Trials (CT) regulations
Approvals continues to prepare for the implementation of the new CT regulations. A series of documents and training will be provided to support the transition to the new regulations. Over the next few months, we will:
- Deliver training at a staff event
- Release an interim internal version of work instructions to enable staff to understand and prepare for the changes
- Provide on-going training between December & March (when the regs are due to be implemented)
Integrated Research Application System (IRAS)
You said:
- Our systems are not intuitive or user friendly, particularly in respect of the navigation
- The questions in the IRAS application seem to be repetitive
- That the document upload process is confusing
We’re working to transform our online systems and bring improvements for researchers and all those who work with us. This includes IRAS, which people use to apply to do, and then manage, health and social care research in the UK.
This work is likely to span the next few years, but we’re focused on making as much progress as we can as soon as we can. If you would like to find out more sign up to our newsletter:
(link: https://public.govdelivery.com/accounts/UKNHSHRA/subscriber/new and then select option for digital updates)
HRA guidance and advice
Guidance for new CT regs - what the new regs will mean for sponsors and how will processes need to change
Working with members of the approval directorate, P&P directorate and MHRA revised guidance has been produced to outline the changes introduced by the new CT regs. This revised guidance was published in October.
REC and CAG Chairs and members' feedback
Between April and September 2025:
- 35 Chairs/Members submitted completed feedback forms to the QA department for the reporting period; all were forwarded to the Health Research Authority (HRA) / Health & Care Research Wales (HCRW) / Devolved Administration (DA) staff for consideration and response.
- The comments received highlighted a range of topics including complimentary comments in relation to staff and chairs and member induction training and mentoring. In addition, comments were received relating to possible improvements to HARP functionality, and a number of negative references to workload, expenses system and the IRAS form.
- All responses to feedback have been sent out for the reporting period.