Performance report
This section provides an overview of the Health Research Authority and our work. This includes our performance highlights and analysis providing details of our key activities over the past year delivering our strategy and statutory functions.
Chief Executive's introduction and performance overview
Over the last 12 months we have seen a huge amount of change in the government and the NHS. The general election put the NHS front and centre, with the new government making a commitment to build a health service fit for the future.
One of the first things the new government commissioned was a report into the current state of the NHS. Lord Darzi’s independent investigation of the NHS laid out some hard truths that were difficult but necessary for everyone working in health and social care to hear.
We were pleased to see that innovation and research were highlighted as key priorities to help make the NHS more sustainable and ensure the UK remains a global leader in life sciences.
I’m proud of the work the HRA has done to support these priorities, and as 2025 marks the final year of our current strategy, we have been working with our stakeholders to develop a new strategy that will set out our priorities for the next 3 years. This will be central to how we support the government's growth mission and align with the NHS 10 Year Health Plan.
Our new strategy will also reflect the feedback we have received from the research community, when we asked what we can do as a regulator to make changes that benefit everyone.
We will of course, be preparing for the new Clinical Trials regulations during the 12 month implementation period before they come into effect in 2026. This will involve developing and publishing guidance to accompany the new regulations to support researchers and stakeholders across the sector.
As part of the UK Clinical Research Delivery programme, we will also be working with others to make a faster, more efficient, more accessible and more innovative clinical research delivery system to make the UK a world leader in clinical trials. This will help us realise the Prime Minister’s vision for the time it takes to get a clinical trial set up cut to 150 days by March 2026 and make an important contribution to the government’s target to reduce the burden of regulation on business by 25% by the end of the current parliament. In addition, the digital transformation of services for research approvals, which will be a key focus in 2025 and beyond, will further support the government’s mission to build an NHS fit for the future.
Throughout the past year, we have also been working hard to develop a replacement for the Integrated Research Approval System (IRAS) and digital services. All researchers in the UK use these services, and we also have a UK-wide partnership to plan, approve, set-up, manage and complete research in the UK.
The new services will digitise the end-to-end research journey, connecting the processes for health and social care research in the UK. They will make it faster and easier to start research by streamlining research approvals, reducing inconsistencies and duplication in study set-up and delivery, and lowering the burden on NHS Research and Development departments.. We are investing heavily in this work which will deliver a world-leading approvals system within the next 2 years.
We understand the importance of a cross-sector collaborative approach to achieve these goals, and we work to bring together stakeholders in health and social care research to maximise our collective impact.
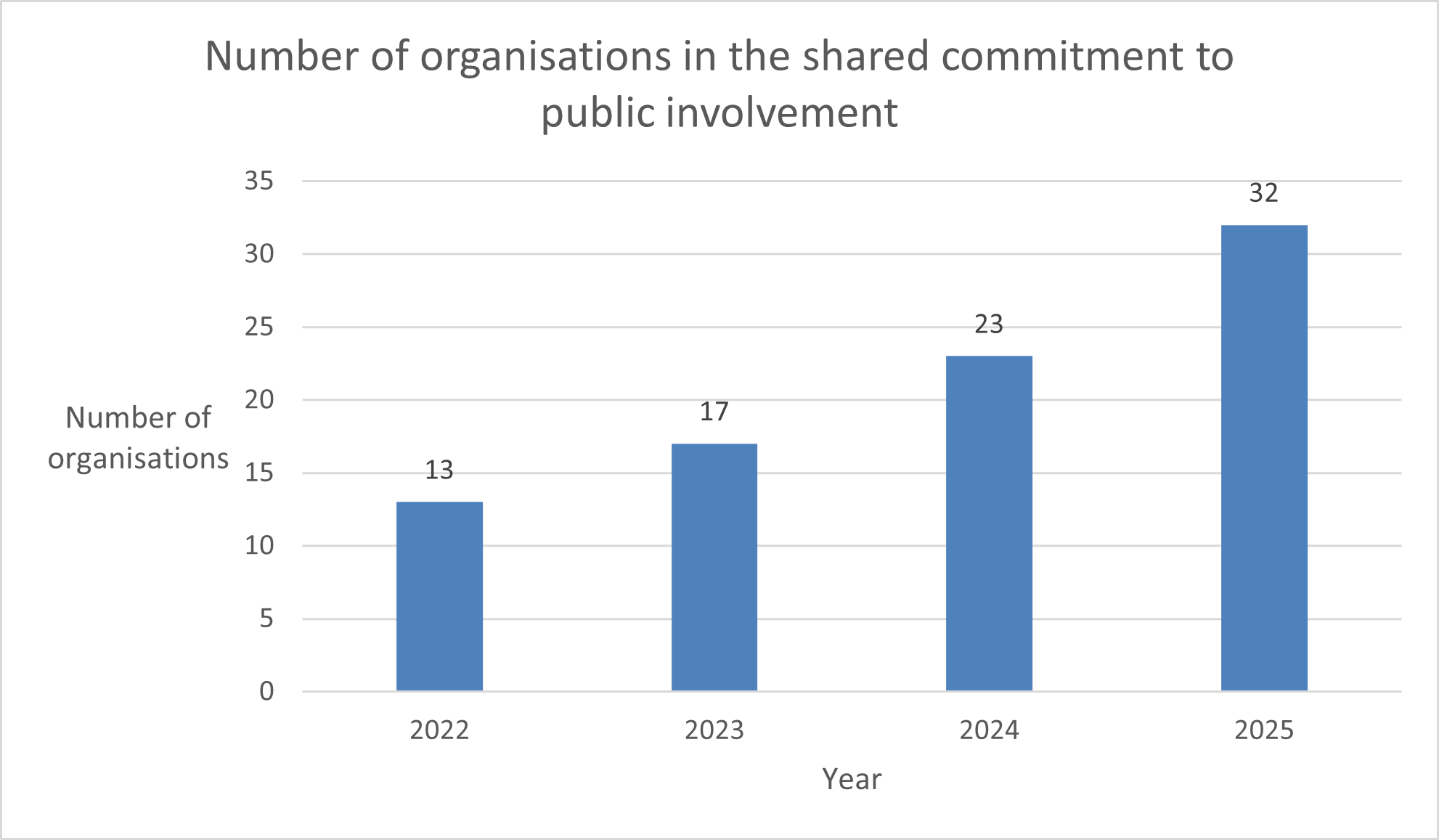
These efforts have meant that we have cut approval and contracting times by 38% in the past year, and alongside the National Institute for Health and Care Research (NIHR), brought together more than 30 organisations to be part of the Shared Commitment to Public Involvement. We also launched a new toolkit to support research that is carried out in more than one country in the UK. We were proud to be recognised by the Institute of Regulation for some of these achievements when we received their Collaborative Practice award in March 2025 on behalf of the HRA and our partners.
In September 2024, the UK was recognised as a country with a strong clinical trial infrastructure in the World Health Organization’s new guidance on best practice in clinical trials. We were pleased to see that the HRA was named in the report as an example of an organisation providing regulatory and ethics approval for research on health and social care.
This has been a key focus for us over the past year, with progress made on updates to the Medicines for Human Use (Clinical Trials) Regulations 2004. In December 2024, the updated regulations were formally laid before Parliament for debate. This was a huge milestone in the process for the change in law, which will further speed up the approval process, enhance transparency and accountability for research findings and reduce unnecessary burdens while upholding ethical and safety standards.
The updated regulations represent more than 2 years of work alongside the Medicines and Healthcare products Regulatory Agency and will be the most significant update to UK clinical trials regulation in 2 decades. It brings us one step closer to the even more vibrant clinical trials landscape that we all want to see, that patients and the public in the UK deserve – and that everyone benefits from.
I want to finish by acknowledging the incredible efforts of staff and our volunteers over the past year.
The Health Research Authority simply wouldn’t be able to do what we do without our volunteers who include our Research Ethics Committee members, Confidentiality Group members, and public contributors. During 2024 alone, our Research Ethics Committee members in England gave more than 75,000 hours of their time for free to review research applications.
As with all government and NHS organisations who have been asked to make savings, we have had to make some difficult decisions. I don’t underestimate the impact that change like this has on staff. There are likely more changes to come in 2025 as the NHS and arm’s length bodies like us continue to transform.
I am grateful for the amazing work they do on a daily basis to help make improvements to the way health and social care research is carried out in the UK.

Chief Executive, Health Research AuthorityDr Matt Westmore
Key issues and risks
As with all organisations, there are risks that we face in the implementation of our strategy and statutory functions. We actively and effectively manage these to minimise any impact on users of our services, as well as other stakeholders and our people. Page 41 gives more detail about our risk management system.
Risks are scored on their likelihood and for the severity of their impact, from 1 (low) to 5 (high), both before and after any actions have been taken to manage the risks. The risk score is calculated by multiplying these 2 scores together, giving a maximum risk score of 25. All risks scoring over 12 after actions have been taken are reviewed by our Executive Committee and all risks scoring over 15 are included in our corporate risk register. Notable risks this year include:
Delivery of transformed and improved research digital service
Risk score after actions have been taken: 20
This risk relates to the transformation of our research digital services, so that it meets the needs of the health research community, making the UK an attractive place to conduct research. Our digital services have multiple connections and dependencies across several organisations and transforming these is a complex programme to deliver.
The programme and HRA have successfully transitioned from set-up to delivery, with increased capability and size in both programme and digital teams. There has also been significant progress gained in creating a digital function to sustain legacy services and prepare for supporting future services.
The successful completion of a Gate Review 0, undertaken in July and August 2024, and the Government Digital Service (GDS) Alpha assessment, undertaken in November 2024, evidence the progress made this year including put in place the recommendations made from these activities.
A key recommendation from the Gate Review 0 related to the refresh to the business case which was approved by the Board in March 2025. A change request to support the revised roadmap was approved by the DHSC Investment Committee in February 2025, subject to Spending Review funding being confirmed and a refreshed Business Case was prepared on this basis which has been approved by HRA Board.
Target Operating Models for both our future research digital services IRAS and the HRA overall are being developed, which will clarify and distinguish between programme delivery and long-term operational requirements.
Successful delivery of our objectives due to financial pressures
Risk score after actions have been taken: 12
We recognise the challenges in delivering our business plan objectives due to increased cost pressures such as inflation, Agenda for Change pay award increases, and uncertainty surrounding the required finances for the successful completion of tour digital transformation. This potential for a shortfall in funding would impact on successfully fulfilling the HRA’s strategic objectives.
To manage this, we have put in place rigorous business and financial planning for 2024-25, identifying the risks associated with budget shortfalls as well as achieving £1.5m reduction in our recurrent expenditure through a cost saving and efficiencies exercise this year to save 10% of annual expenditure.
Recruitment and retention of an effective workforce to meet our objectives
Risk score after actions have been taken: 16
The current employment market combined with competition in the public sector for specialist skills and capabilities, such as technological roles, is making it difficult to recruit to vacant positions, increasing pressure in attracting and retaining staff. This scarcity of suitable candidates for positions combined with our cost saving exercise has resulted in under-resourcing, impacting on our ability to deliver our business plan.
We are planning to revise our People Strategy during 2025 and 2026 which will focus on improving our attractiveness to candidates as well as retaining key capabilities. This risk is currently mitigated by the HRA offering strong employee benefits and total reward packages as well as maintaining good satisfaction scores from annual staff surveys.
Performance analysis
Performance management
We plan our work in order to achieve our strategy and statutory functions. We do this by preparing an annual business plan, which sets out how we will deliver our strategy and our statutory functions. This plan is prepared with the involvement of a wide stakeholder group, and is embedded throughout the organisation in our performance, people and risk management processes.
These processes help to make sure we successfully achieve our strategic goals and meet our statutory functions. Importantly, it also helps our people understand their role in delivering our plans.
We monitor and evaluate our performance against our strategy, business plan and financial plan every 3 months, and we collate a comprehensive performance report for our Executive Committee and Board to review. This includes focused performance metrics including user satisfaction data, an analysis of our change portfolio, a detailed performance report, risk management report and finance report. These reports combined provide assurance on how we are delivering our strategy, statutory functions and highlight areas for action and improvement.
Individual staff objectives that complement and support these organisational objectives are developed during the annual appraisal process and monitored throughout the year during regular one-to-ones between staff and their managers.
The way that we manage our performance and its relationship with risk and uncertainty is explained in more detail in our corporate governance report on page 34.
As a learning organisation, we regularly review and refine our performance management and reporting systems, to ensure we continue to fulfil our strategy ambition and statutory functions. We do this through continuous improvement activities such as lessons learned exercises as well as independent assurance activities including internal audit reviews.
We also set operational key performance indicators, which are collated and monitored by delivery teams, making sure we are achieving our statutory requirements as well as continuously improving our services. Operational metrics are reported every 3 months to our Executive Committee.
Performance reporting
Our performance reporting focuses on 4 areas:
- customer satisfaction
- our services performance
- our people
- financial performance
User satisfaction
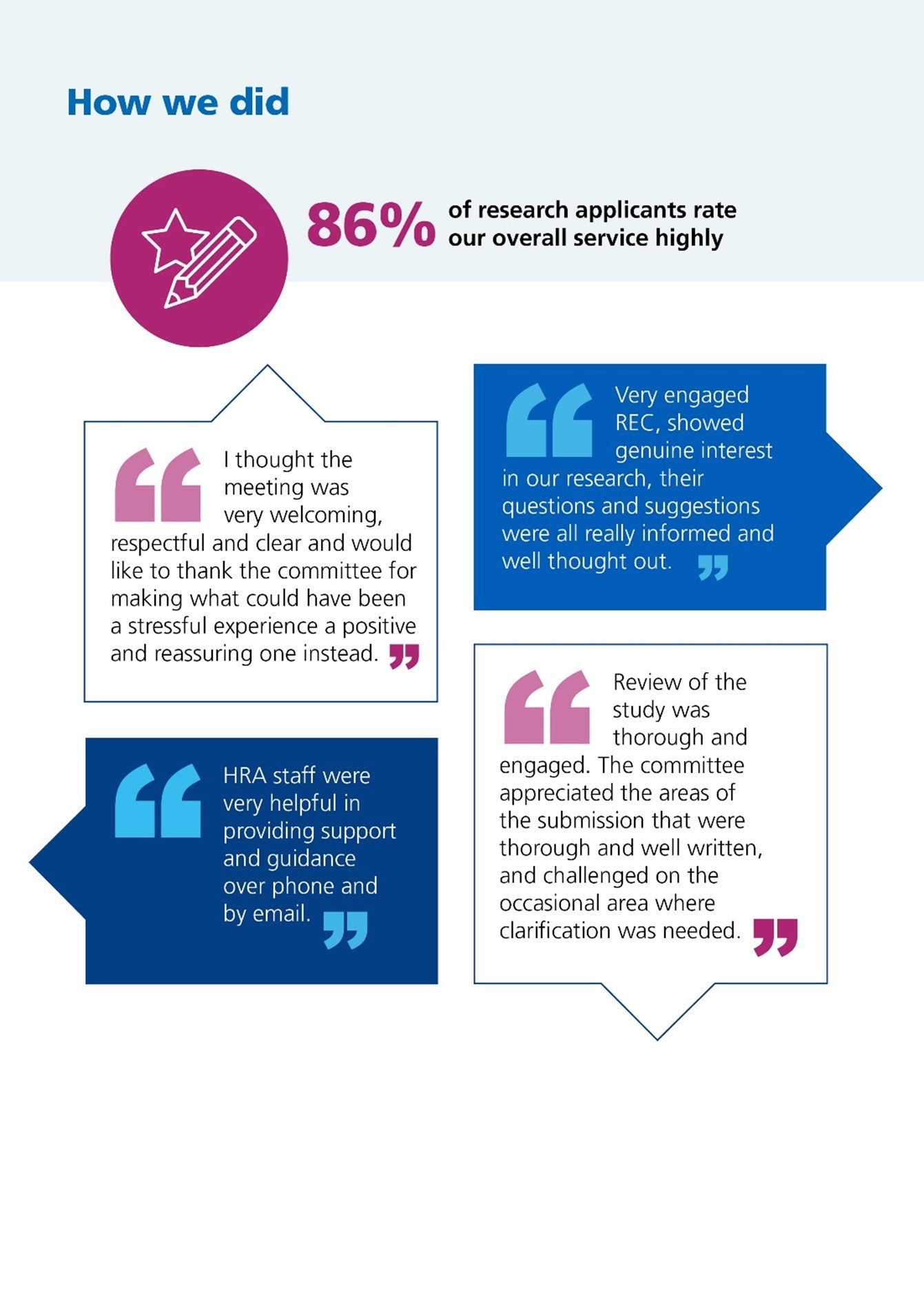
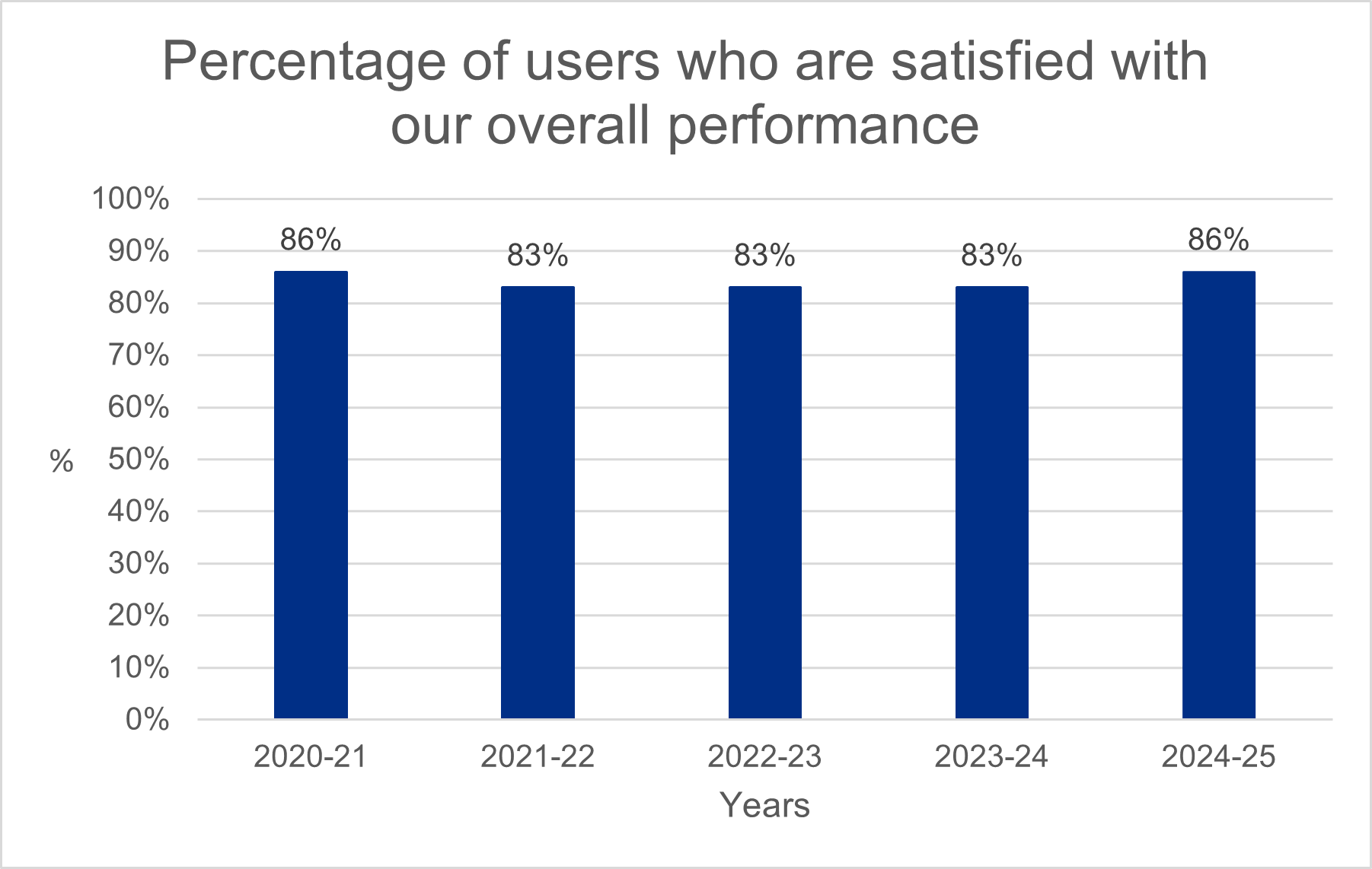
We measured the satisfaction rates with our services, gathering data from those who use them. We’re pleased that satisfaction with our overall performance has improved this year and has exceeded our 75% target.
Our staff continue to score the highest in our satisfaction surveys, with our digital research systems scoring the lowest. We have used this feedback to inform our digital transformation which is focused on improving user experience, making it easier and faster to set up health and social care research in the UK. We expect to begin to see these scores improve in 2026-27.
Our services performance
One important measure of our performance is the time it takes to conduct ethics review of clinical trials of investigational medicinal products (CTIMPs). Ethics review is provided by Research Ethics Committees (RECs) that assess research applications to determine whether they meet ethical standards. Our statutory performance target requires all ethical reviews of CTIMPs to be performed within 60 days. This graph sets out the percentage of reviews that were performed within this target. This year, for the first time, we have met our statutory performance target throughout the year. This is a fantastic achievement and exactly what the research community told us they needed; a predictable and fast service they can rely on.
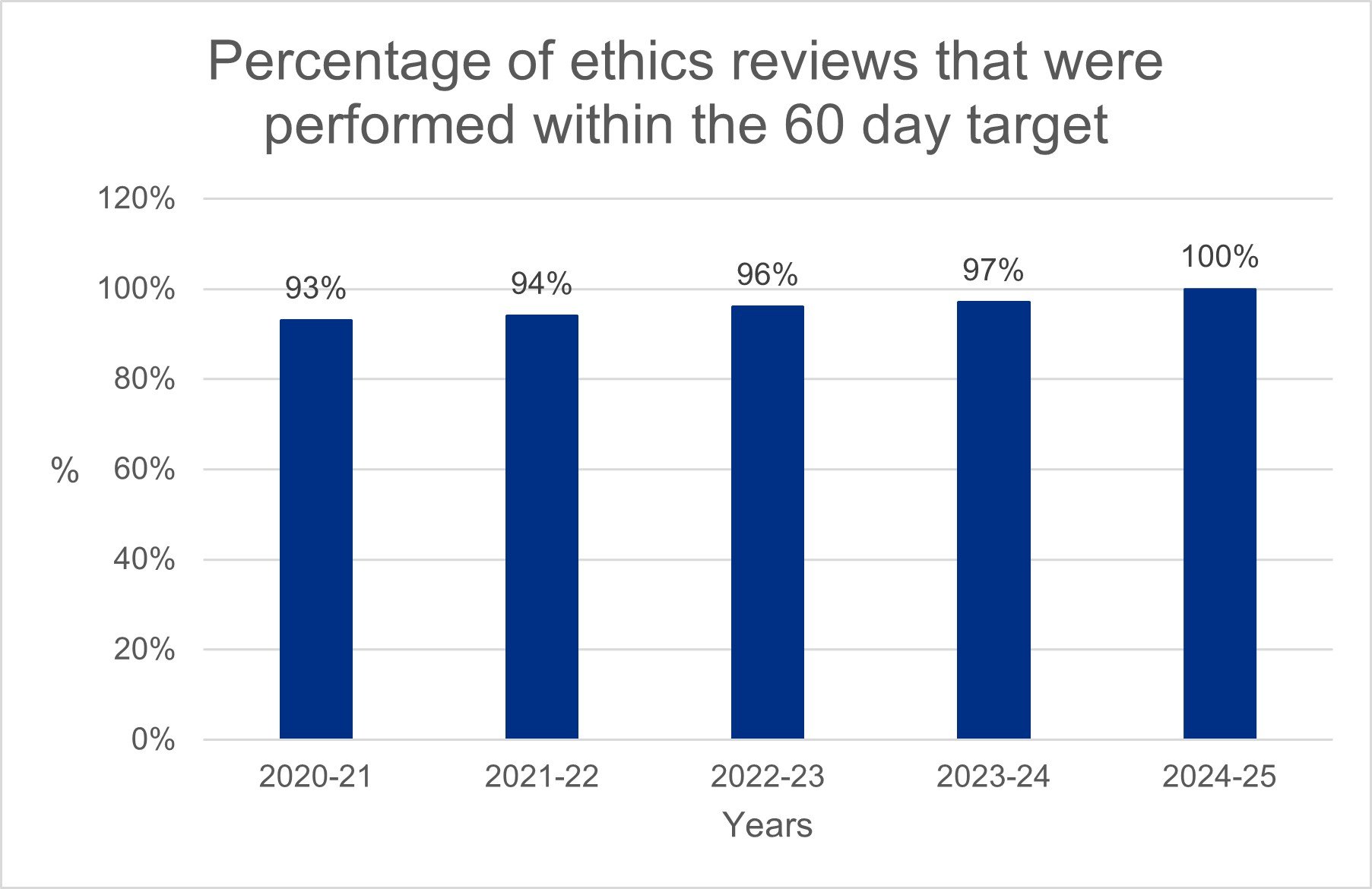
This year 4% of these reviews resulted in an unfavourable opinion. This is consistent with our previous experience.
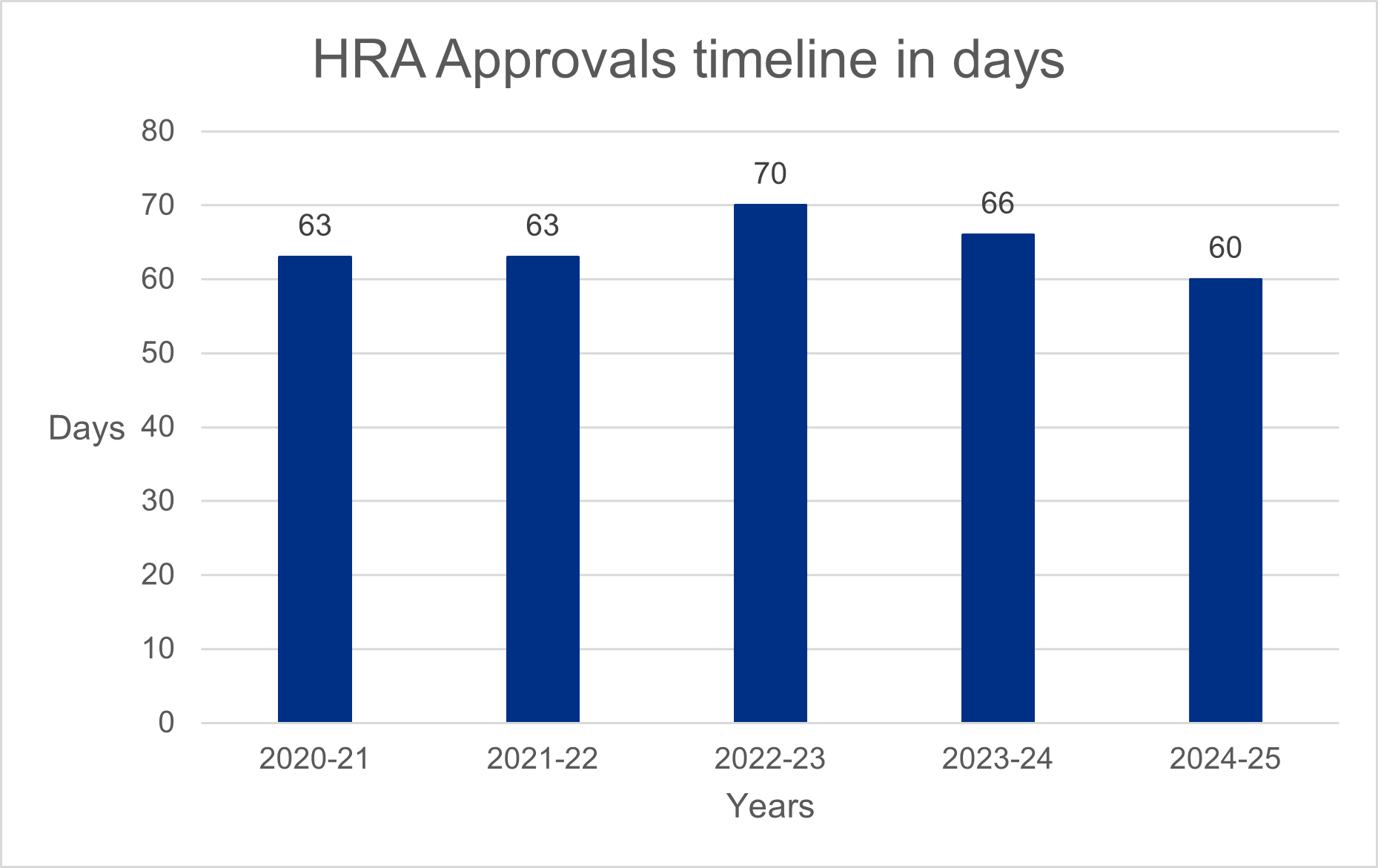
We also measure our performance by the time it takes to deliver our HRA approval service. This service brings together the assessment of governance and legal compliance, undertaken by dedicated HRA staff, with ethics review, performed by a Research Ethics Committee. This timeline is not completely within our control as parts of the process rely on other review bodies and coordinating organisations to deliver a combined outcome to researchers. We work collaboratively with these organisations to make sure timelines are predictable and streamlined.
This year we have reduced the time it takes to deliver this service, with 100% of applications processed within 60 days for the first time. This is a significant success for the HRA, following several years where timelines had been extended due to delays experienced by the research regulator Medicines and Healthcare products Regulatory Agency (MHRA).
Our people
Our staff and HRA Community members have continued to demonstrate an outstanding commitment to UK health and social care. Our people make sure our statutory services are on time and consistently of high quality. They are also key to implementing our strategic goals. We measure capacity to help us understand where our services may be under pressure so that we can put mitigations in place to maintain good services. We measure both staff and committee membership capacity to:
- compare our actual staff capacity compared to planned levels
- monitor our committee membership vacancy rate
Staff capacity
We plan our work to achieve our strategic objectives and provide our essential statutory services. This includes a careful assessment of our staffing, their skills and capabilities to make sure we have sufficient capacity to make our plans happen. This year our staff numbers were significantly below our planned levels. This has limited our ability to focus on continuous improvement activities, with some being deferred to 2025-26 while we make sure we deliver on our statutory functions and digital transformation.
This low capacity was due to several factors:
- a cost saving and efficiency exercise which included a recruitment pause, purposefully reducing our capacity to manage funding pressures and enabling a change process to be successfully implemented
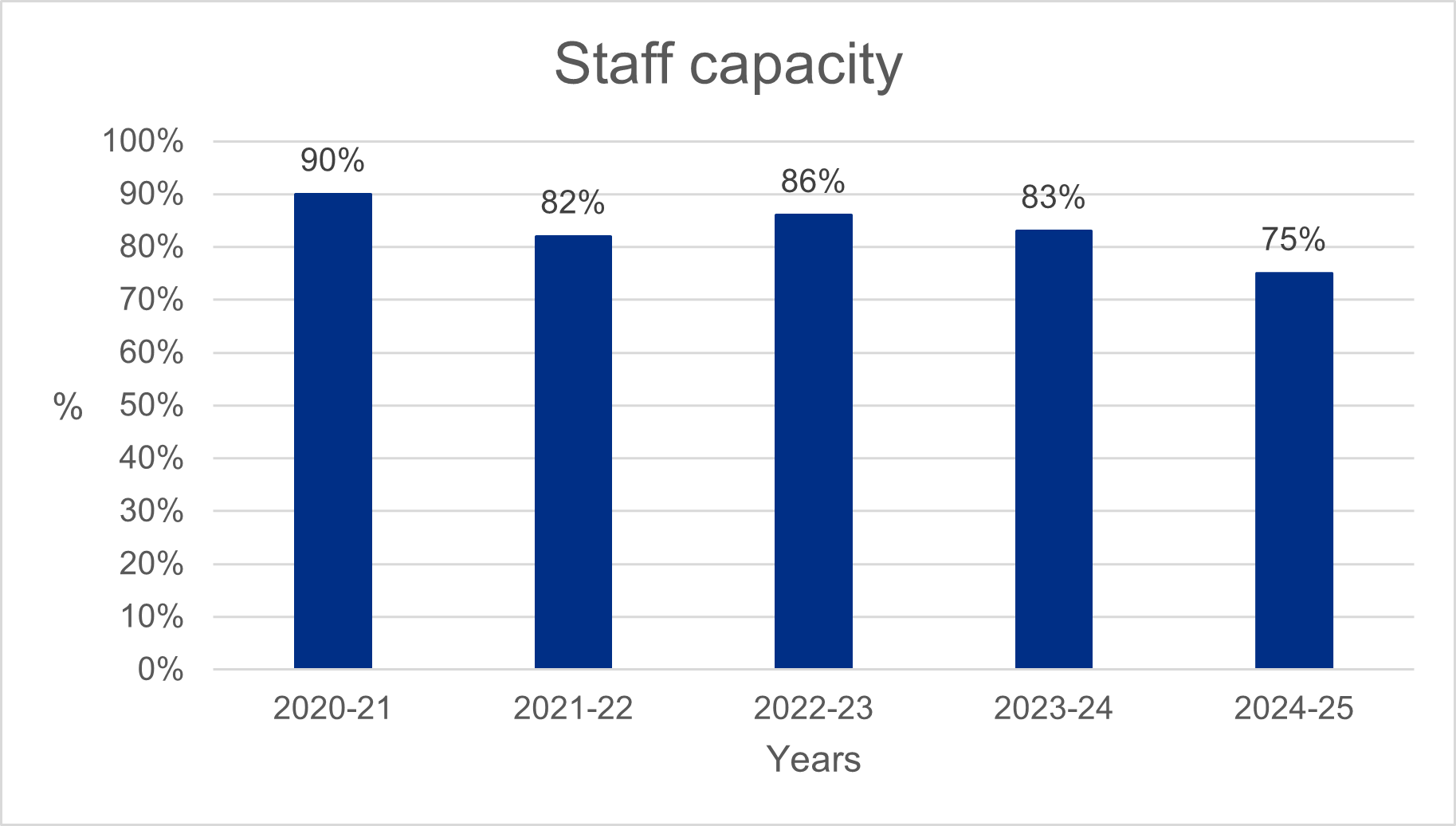
- delays recruiting to roles to enable our digital transformation. Tough market conditions made it difficult to attract and retain people with much-needed specialist technical skills
- funding uncertainty for our digital transformation impacted on our ability to offer attractive terms of employment
We have put in place several actions to improve staff capacity, including adapting our recruitment approach to better reflect expectations in the market, revising job descriptions to reflect professional standards, offering recruitment and retention premia where appropriate as well as offering internal development opportunities to grow our in-house capabilities and capacity. We have also redeployed many staff impacted by the cost saving restructuring this year, retaining their valuable skills and expertise within the organisation.
It is good to note that we have started to see the gap between our planned and actual staff numbers reducing. We are also putting in place additional monitoring for our Executive Committee and Board to make sure we continue to improve our capacity to make sure we deliver our strategy and statutory functions throughout 2025-26.
Committee membership vacancy rate
The committee membership vacancy rate has improved significantly compared to previous years due to a strengthened recruitment campaign, simplified application process and improved internal coordination of interviews and appointments. This has resulted in our vacancy rate being within target for most of the year.
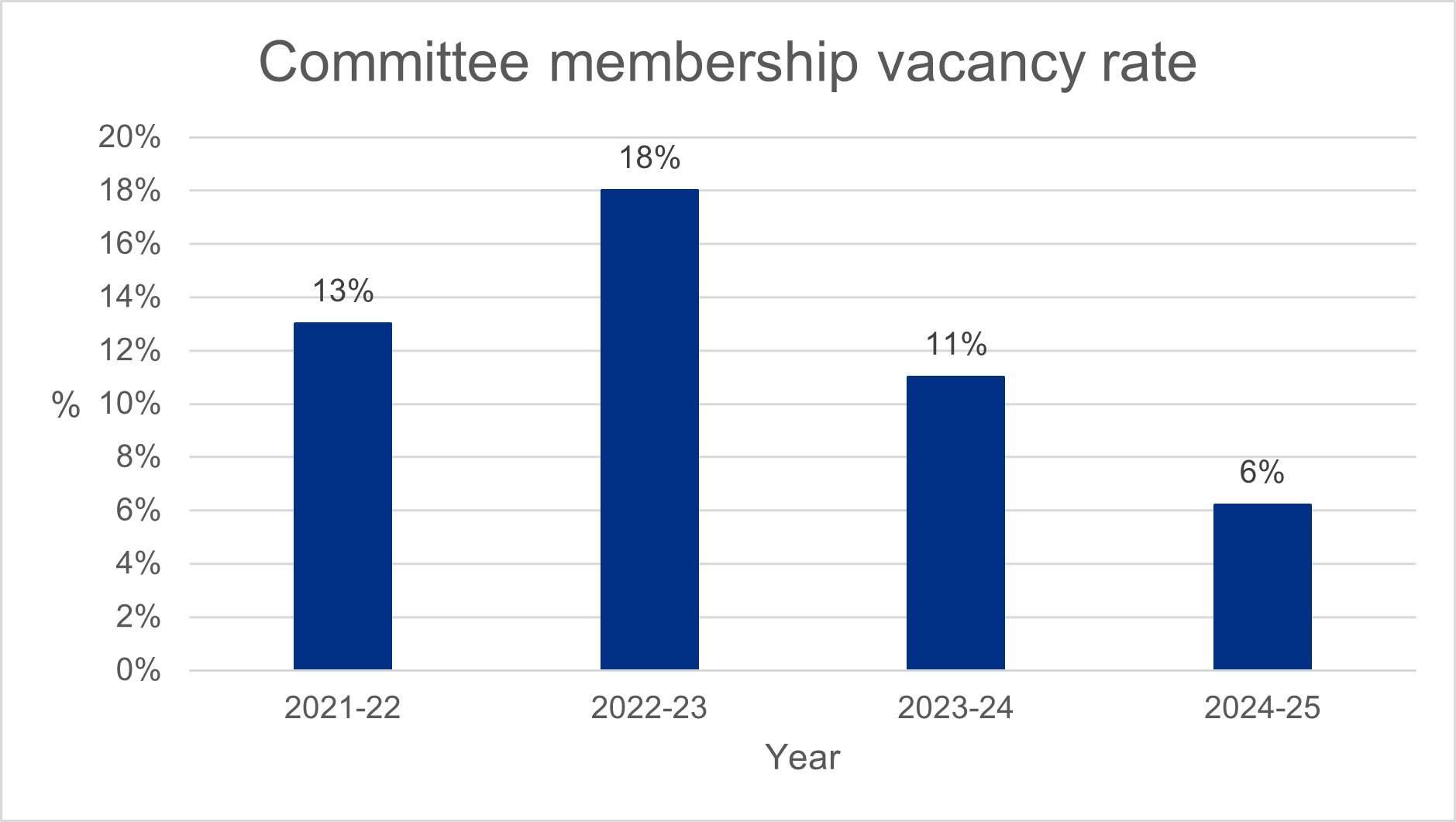
Recently we have had to pause our recruitment activities while we put in place process changes to meet Home Office guidance as well as streamline our systems while we manage funding pressures. These additional activities have meant our recruitment activities have been delayed, having an adverse impact on our vacancy rate which increased in February to 10%. We are pleased to see this has now been reduced to 7% and within our target range of 8% on 31 March 2025.
Financial review
Our accounts consist of our primary statements providing summary information about our income and expenditure in the year, our assets and liabilities at the end of the year, and how we have managed our cashflows. They also include detailed notes to these statements that provide more information about our accounts.
Our accounts have been prepared based on the standards set out in the Government Financial Reporting Manual (FReM) to give a true and fair view. We remained within our agreed revenue and capital funding allocations for the Department of Health and Social Care (DHSC) for the year-ended 31 March 2025.
Government funding
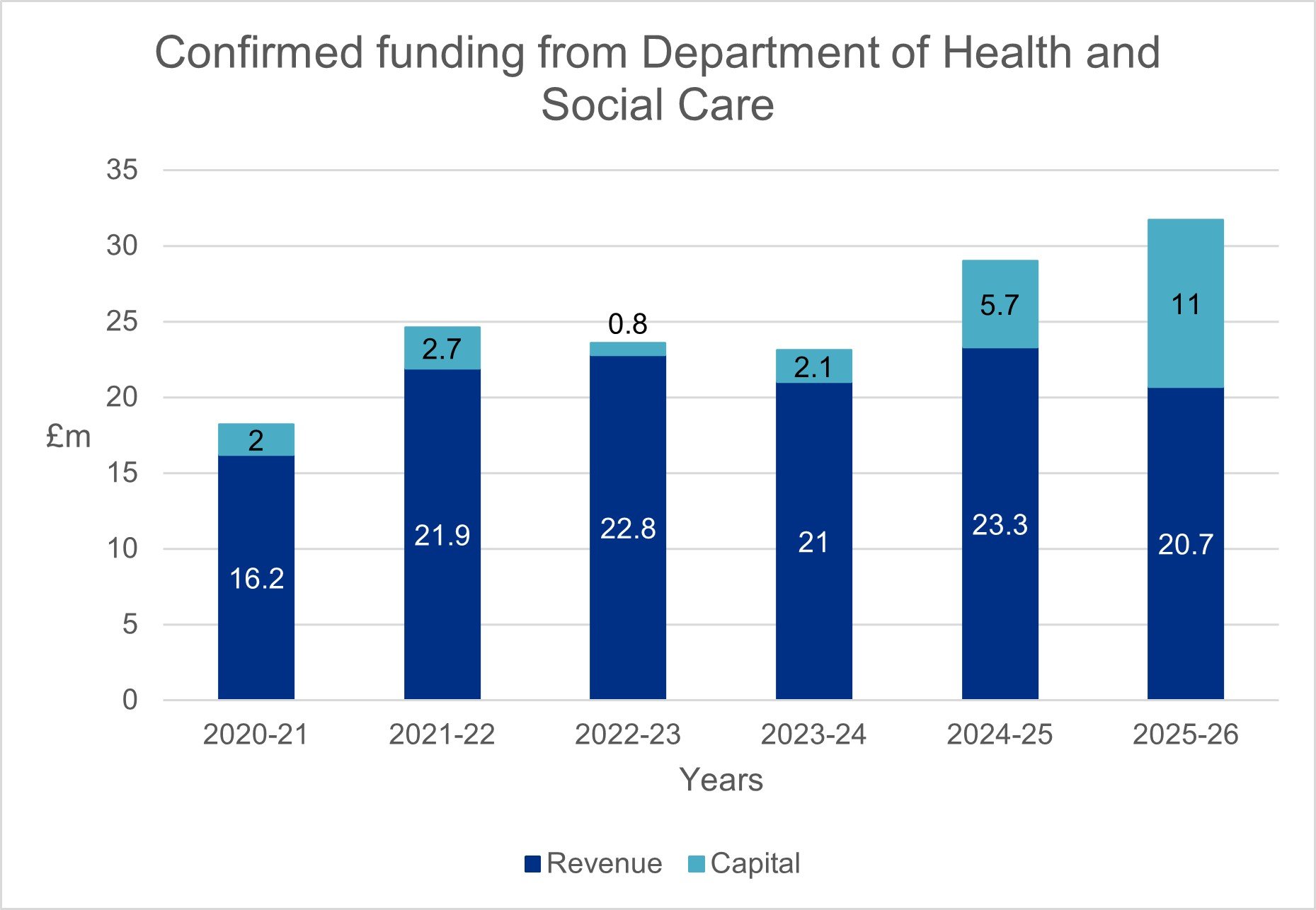
Our total confirmed funding from DHSC for the year was £29.0m (2023-24 £23.1m), of which:
- £23.3m (2023-24: £21.0m) was revenue funding
- £5.7m (2023-24: £2.1m) was capital funding
Funding received from DHSC in year was £24.8m (2023-24 £19.6m) due to efficiency savings and underspends related to our digital transformation.
This year, to manage unfunded inflationary pressures and to create capacity to invest in our digital transformation we introduced a savings and efficiency project to achieve £1.5m reduction in our core costs. These changes were achieved through many initiatives including reducing our property requirements, stopping non-statutory services, changing team structures and making better use of public sector shared services. The project was completed earlier than anticipated and savings achieved in year reduced our total expenditure and related funding. These savings combined with the increase in confirmed funding in 2024-25 and 2025-26 will enable the replacement and transformation of our research digital services. This activity will strengthen the UK’s competitive position in global life sciences and support the NHS in delivering important research by enabling easier, faster and more cost-effective research approvals. The case for funding has been approved by DHSC Investment Committee. Continued funding for the Programme beyond 2025-26 is dependent on the Spending Review.
The uplift in funding between 2020-21 and 2021-22 enabled the successful launch of our combined review service in collaboration with MHRA. This new service, introduced in January 2022, allows researchers to apply for both ethics and regulatory approval in a single application, streamlining the process and, on average, reducing the time it takes to gain approval by 50%.
In 2022-23 we decided to pause our digital transformation to review our approach and learn from implementing combined review. This pause reduced the funding we needed in 2022-23 and 2023-24 to undertake this programme, particularly capital funding. We agreed, as part of the DHSC Efficiency and Reform Review, to defer activity while we made sure the Programme was set up for success, reducing in year programme costs. The programme was relaunched in Autumn 2023 and funding has increased as a result.
Other revenue
We also received income from cost-sharing arrangements with other publicly funded organisations, which included:
- £0.39m (2023-24: £0.43m) from the devolved administrations for providing support and digital systems to deliver the UK research ethics service
Future funding
The Department of Health and Social Care have confirmed our revenue funding for 2025-26 is £20.7m and our capital funding is £11.0m.
How do we spend our funding?
We take great care to plan our activities well to deliver our strategy and statutory services. Our business planning process appraises how best to invest our limited resources to provide our statutory functions as well as to implement our strategic plan. This process makes sure we spend our funds wisely, maximising our impact, based on our confirmed funding. During the year, as we put in place our plans, we may not draw down all this funding particularly if our plans are changed, delayed or we achieve savings.
Our total expenditure for the year was £20.2m (2023-24: £18.5m), resulting in an underspend on our committed revenue funding of £3.5m. This underspend was mostly due to delays onboarding capabilities to enable our research systems transformation resulting in lower staff costs, lower than anticipated licencing costs and a changed approach to VAT following an independent review. In addition, we achieved savings earlier than planned through our efficiencies project, reducing our expenditure in year and amortisation costs were also lower than planned, reflecting the changed roadmap for the Programme. Most of our expenditure (76%) is staff costs at £15.3m (2023-24: £13.7m, 74%). Our staff are paid in line with agenda for change pay scales and associated terms and conditions. Our staff structure is agreed as part of the annual business planning process. Any changes to this agreed structure are governed by our scheme of financial delegation and business case process.
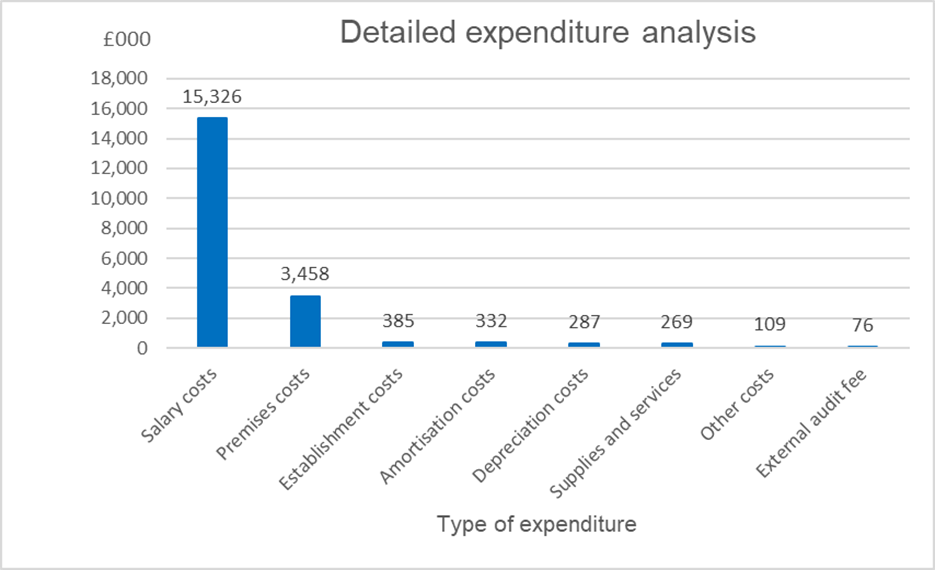
Making payments
We aim to comply with Better Payments Practice Code by paying suppliers within 30 days of receipt of an invoice. The percentage of non-NHS invoices paid within this target was 98.8% (2023-24: 97.4%). This improvement is the result of our public involvement and finance teams working together to simplify and streamline the way we make payments to public contributors.
| 2024-25 number | 2023-24 number | |
| Total non-NHS trade invoices paid in the year | 893 | 977 |
| Total non-NHS trade invoices paid within target | 882 | 952 |
| Percentage of non-NHS trade invoices paid within target | 98.8% | 97.4% |
| 2024-25 number | 2023-24 number | |
| Total NHS trade invoices paid in the year | 94 | 104 |
| Total NHS trade invoices paid within target | 94 | 101 |
| Percentage of NHS trade invoices paid within target | 100.0% | 97.1% |
|
2024-25 Value £000 |
2023-24 Value £000 |
|
|
Total non-NHS trade invoices paid in the year |
8,092 | 4,416 |
|
Total non-NHS trade invoices paid within target |
8,092 | 4,374 |
|
Percentage of non-NHS trade invoices paid within target |
100.0% | 99.0% |
| Total NHS trade invoices paid in the year | 1,917 | 1,445 |
|
Total NHS trade invoices paid within target |
1,917 | 1,393 |
| Percentage of NHS trade invoices paid within target | 100.0% | 96.4% |
Strategy performance analysis
This section covers how we have performed against the 4 pillars of the HRA strategy, as we come to its end and prepare to launch our new strategy in 2025. We cover activity that aligns with each of the commitments under each pillar and detail our achievements as well as where there is still work to do.
Include: health and social care research is done with and for everyone

Include everyone in research
We want to make sure that clinical research can improve the health of the whole population. We are working with MHRA to help researchers improve the diversity of participants in their research to help make this happen. To do this, we are developing a set of questions and supporting guidance for researchers to consider when they design clinical trials and clinical investigations. The answers to these questions will form the basis of an Inclusion and Diversity Plan. This will help ensure clinical guidance is designed to include people who could be impacted by the findings, and that people underserved by research are not overlooked.
The questions and supporting guidance have been created in collaboration with members of the public and the research community.
The Health Research Authority, the National Institute for Health and Care Research (NIHR) and a host of organisations across the UK have been working together to bring about changes which will drive up standards in health and social care research, as part of a Shared Commitment to Public Involvement. This year, we celebrated our third anniversary, increased the number of partners involved to more than 30 organisations, and held learning and sharing meetings. We also wrote draft guidance on public involvement in clinical trials, including guidance focused on healthy volunteer trials.
Promoting transparency across the research landscape is central to our role to facilitate safe and ethical research. Our aim is that trusted information from health and social care research studies is publicly available for the benefit of all. Research transparency is central to ethical research practice. When research is carried out openly and transparently, everyone will be able to see what research is happening and the outcomes from finished studies.
#MakeitPublic is a campaign dedicated to research transparency with a focus to reach 100% registration of UK clinical trials. It is led by a campaign group which includes experts from across the sector and members of the public. This year, to improve research transparency, we wrote and published guidance to increase transparency of clinical trials of medicines. This included guidance for registration, publication of results and feeding back to participants.
We also developed ways to assess how well researchers are making their research public and have continued to collate data on rates of registration compliance for clinical trials approved by a REC. Our 2023 report shows 92% of studies are registered or in the process of being registered, and highlights those organisations who have not registered.
This year, we planned to update the UK Policy Framework to reflect the new clinical trials regulations. This did not happen due to delays to the new regulations. The work has now been planned to coincide with the implementation of the new regulations in 2025-26.
Ask you what you want research to look like and act on this
This year we published a report that makes a series of recommendations for how to improve clinical research for participants. This report was the result of our People-Centred Clinical Research Project completed in 2024, in partnership with members of the public, researchers and academics from the University of Lincoln which asked more than 400 people about their experiences of health and social care research. We found that there were barriers that can stop research from being people-centred, as well as things that can help. We grouped these under 4 headings and made 19 recommendations for actions that researchers can take.
We used the findings of our public attitudes towards research survey of over 5,000 people from across the UK to inform our work this year including the design and content of our REC development days, external engagement activity and in promoting the importance of research transparency, public involvement and sector-wide efforts to improve participation in research.
We also built relationships with individuals, groups and communities we had not previously worked with to increase our understanding of what matters to them to inform our work. This included meetings with the Caribbean and African Health Network and NIHR Applied Research Collaborations (ARC) Young People’s Advisory Group.
We grew knowledge of our work and why it matters by increasing our presence at national and international events run by key external stakeholders such as Future of Life Sciences, Westminster Health Forum, Clinical Outsourcing UK Conference and Decentralised and Hybrid Trials World Conference.
We engaged with a diverse group of stakeholders, including the public, to inform how to simplify the way that researchers seek and document informed consent in a way that people can trust. As part of this work, we completed a survey, analysed the results and used these to inform our guidance and online tools.
We sampled nearly 4,000 studies which received a favourable opinion from a REC in 2023, which showed that overall, 74% of studies told us that they had involved patients and the public in their research. Our analysis showed that although there were some great examples of public involvement, the approach is not consistent across all types of research. The data from this report will be used to create a baseline to measure the impact of our efforts to improve the extent and quality of public involvement in health and social care research.
We analysed our data to inform how we push for change, telling evidence-based stories to raise the profile of what we do and why it is important. We analysed trends in ethical issues considered by Research Ethics Committees (RECs) however, we paused developing this work further due to capacity issues.
We sought funding for a public conversation about how people can trust the way that they will be treated if they lose capacity while taking part in a longitudinal research project. Unfortunately, we were unable to secure sufficient funding from partners this year but will continue to support them to take forward this work.
Involve you in the HRA
We want to make sure that health and social care research is done with and for everyone. The HRA Community is made up of almost 1,000 dedicated people who give their time to support the HRA’s work. They make up our:
- Research Ethics Committees (REC)
- Confidentiality Advisory Group (CAG)
- public contributors
This year, we created more opportunities for people to be involved in our decision making through our HRA Community Committee and completed an effectiveness survey to improve how we support this committee.
We supported staff to meaningfully involve people in their work by providing tailored support and the right resources by publishing a handbook to support staff to do this well.
We published demographic information on the current HRA Community and developed an HRA community survey action plan which includes changes that will support a greater diversity of people working with us.
We developed plans for recruitment campaigns for new REC and CAG members that seek to reach people that we do not already work with, and we gave more people the opportunity to see our community in action by observing REC meetings. It was good to see over 800 requests received and to help make this happen, we streamlined our processes so that requests were distributed across all committees.
We made sure our communications channels and products work for the people we want to talk to. This included a review of our social media and the development of a social media strategy.
We also continued to develop the HRA’s voice developing and testing new HRA brand resources such as a new visual identity and tone of voice and style guide. The new tone and style guide are now live.
Accelerate: research findings improve care faster because the UK is the easiest place in the world to do research that people can trust.
Save money and time so that you can focus on doing good research
For all clinical trials and clinical investigations, it is expected that a signed agreement between the sponsor and the host organisation will be in place before the research commences at the site. This year we led UK-wide development and maintenance of a suite of model agreements making it easier and more cost effective to set up research. We maintained existing model agreements, and published new model agreements including one for advanced therapy studies.
As part of work to speed up commercial research in the UK we launched National Contract Value Review (NCVR) for Advanced Therapy Medicinal Product trials and early phase trials. The NCVR is a standardised, national approach to costing for commercial contract research. A standardised approach streamlines and speeds up research study set-up. Over the 3 years of our strategy, NCVR has made a substantial impact on the time it takes to set up a clinical trial which is now taking 6 instead of 10 months.
We also published updates to General Data Protection Regulation (GDPR) standard wording for participant information and developed guidance on in-vitro diagnostic device studies published with MHRA.
We continued to enable more proportionate approval of clinical trials of medicines by introducing a new streamlined notification scheme for certain clinical trial initial applications and amendments with MHRA. This scheme simplifies and speeds up the approvals process, building on our joint experiences streamlining approvals with our combined review service. We also wrote and published guidance on simplified consent for low-risk clinical trials to support the implementation of the Clinical Trials Regulations.
We worked with the Experimental Cancer Medicines Centres to develop technical assurances for clinical trials of medicines. The ideal paths for pharmacy and radiation assurances continued to be improved in collaboration with partners. We also drafted guidance for pharmacy assurances and planned a pilot for this service.
To help make it easier and faster to set up research in the future, we began to design a high-level research study set-up path and tested this with stakeholders. The aim will be to find the most efficient way to setup research studies, making it easier to do research in the UK and ensuring that everyone involved in the process understands each step. We also continued to integrate our own approvals services to improve user experience by simplifying the coordination of our ethics review and confidentiality advice services. We are keen to continuously improve our services whilst also looking to the future to redesign the entire research study set up process.
Create a new online system to help you make research happen
Our Research Systems programme is a large digital transformation programme, with a vision to:
- connect processes for health and social care research in the UK
- help people work together to plan, approve, set up, manage and complete research
- help make the UK be a great place to do research that people can trust
We are developing new services that will digitise the end-to-end research journey, allowing users to plan and prepare new research, make changes to existing research and review and approve research applications in a seamless way. We will also improve the support offered to users.
We are leading the development of the new integrated service on behalf of the Integrated Research Approvals System (IRAS) Partners. We are working closely with them and other stakeholders to collaborate on designing the new services.
The new services will deliver better user experiences, offering more integrated and seamless services for the whole research community. Meeting user needs is at the heart of the improved services we are developing, and we are involving users at every stage. As well as significant user research, our designs respond to the key issues users have told us they experience with the current systems.
This year we worked with IRAS Partners to agree a vision for our digital services, set up our programme for success by embedding agile methodology and positively responded to Gate Review 0: Strategic Assessment recommendations.
We also completed the GDS Alpha assessment with a positive outcome (12 green and 2 amber), confirming delivery largely meets expected standards, and that we could proceed to the private beta phase.
We agreed an updated version of the programme roadmap and worked with IRAS Partners and users to define the requirements and test designs for ‘the make changes to approved research’ and ‘review research’ services. We also explored how to effectively support researchers to navigate the end-to-end research journey, with guidance and through digital tools. This included consideration on how guidance is presented and managed in the service.
Support new ways to do research
We worked with others to enable more people-centred research by considering decentralised research models to widen participation and enhance flexibility for research participants. We collaborated with Industry and interested stakeholders to work together to look at opportunities to improve research set-up in non-traditional settings. We outlined the governance barriers to setting up research in this way and made proposals to DHSC to support the development of regional delivery models to help remove these barriers
We created and maintained links with stakeholders to understand their needs and through user-centred design harnessed opportunities for innovation and change.
We worked together with the National Institute for Health and Care Excellence (NICE), MHRA the Care Quality Commission (CQC) and with the NHS AI Lab to provide the AI and Digital Regulations Service (AIDRS). This service helps guide developers and adopters of AI and digital technologies through the often complex legal and regulatory areas that apply to emerging technologies, particularly AI. This year, we maintained content on the website so that our guidance remained up to date.
Design our digital technology to do our work well
We received a positive outcome when we presented an updated change request to DHSC Investment Committee for our Research Systems programme which secured funding to continue for 2025-26. Funding beyond 2026 is dependent on the Spending Review. We also agreed a business case to improve customer service management through consolidation of our service desk functions and creating a better digital user experience. This work is expected to yield benefits in 2025-26.
We created a supplier management plan to support our legacy research systems and to help make sure they remain resilient while we transform them. We held regular meetings with legacy suppliers to ensure delivery of HRA digital and service objectives and services remain available.
As part of this resilience work we also reviewed and updated our incident and request management and major incident processes including the development of a service catalogue. This included developing workflows with our current service desk supplier in preparation for the new service desk and developed templates for more automated ticketing operations and dashboards for reporting. This has helped us understand better the services we provide and how we can improve them. We also tested our incident response plan so that we know we can respond quickly and effectively if there is an incident.
Resilience also requires robust cyber security processes. We continued to improve our approach by effectively implementing essential controls and undertook a Cyber Business Continuity Exercise which demonstrated that we have strong capability and controls in this area.
We made the most of our learning management system (LMS) following its successful implementation in 2024 with increased engagement and user satisfaction with both staff and volunteers. 57% of staff responded in our staff survey that there were opportunities to develop their knowledge, skills and behaviours at the HRA, an increase of 22% on 2024 (35%).
Always look for ways to do things better
Always learn, improve, and innovate
We completed a review of our internal communications, making sure that staff always have access to the information that they need and it is easy to find. This included the launch of our new intranet which has resulted in a 35% increase in staff confirming that the intranet is an effective communication method (74% compared to 39% in 2024).
We completed an organisation-wide skills analysis and training needs assessment in addition to a capacity and capability internal audit review. We also reviewed and improved our approach to secondments and updated our secondments guidance and policy.
We developed and put in place continuous professional development (CPD) programmes including a ‘90 minutes to learning’ pilot and the introduction of NHS Elect, resulting in 25% improvement in staff confirming that they had equal and fair access to learning and development (87% compared to 62% in 2024).
We continued to grow a culture of innovation and change at pace to support our change portfolio including our digital transformation. We developed a Research Systems programme change management strategy, transition states definition and Future IRAS target operating model design approach.
To meet best practice, we improved the way we buy and pay for goods and services by implementing a new purchase order policy and made improvements to our portfolio, programme and project management processes. This included an updated business case process, senior responsible owner training, improved prioritisation, dependency mapping and capacity and resource management.
We reviewed and improved how our online Research Ethics Committee (REC) meetings are run and ran a programme of learning as part of the National Chairs Day in March 2025. We continued to develop good practice standards and mechanisms for the ethical review of research applications with a focus on ethics theories, diversity, and participant information standards.
We also introduced improved governance when recruiting volunteers to our committees with clearer terms and conditions as well as identity and right to work checks as part of our initial checks.
Be a great place to work
This year we have continued to see high levels of staff engagement (75%) better than the independently provided public sector benchmark (69%) however levels have reduced by 5% on last year. Over the next few months, we will explore why this is and put in place plans to help reverse this trend. Our response to our 2024 staff survey focused on several key areas:
- valuing people
- improving people’s experience
- developing people
We put in place arrangements to help manage change better, including adopting multi-disciplinary teams, change workshops for managers, improved support for people going through change and open, transparent communications. This has resulted in an improved staff survey score for change being managed well of 41% (compared to 33% in 2024). We implemented several actions to make decision making at the HRA more transparent including widening access to meetings and better communication of decisions made. We also put in place Challenge and Change listening circles and agreed a set of actions to implement to improve people’s experience of working at the HRA.
We involved and engaged with a diverse group of stakeholders and people who bring different experiences and perspectives to develop the HRA's next 3-year strategy which will be published later in 2025. We also supported and embedded principles of Respect at the HRA. This included a Respect video as part of the induction process and new starter workbook, ‘Working at the HRA’ web page, and publication of a new policy suite.
We reviewed and improved our approach to supporting the mental health of our staff by updating our mental health framework and organising refresher training for our mental health first aiders. We also embedded clearer ways of working to support staff working with vulnerable patients, research participants and volunteers. This included developing a policy and procedure that has been reviewed by our Community Committee.
We improved Research Ethics Committee members’ experience by including access to NHS discounts and long service awards, and continued to offer support such as access to development days and learning and development opportunities throughout the year.
Strategic workforce planning did not take place this year as planned. This is due to several factors including our cost saving project Adapt and Change restricting our capacity to perform this work and later our decision to prioritise developing a cohesive, end-to-end framework connecting our new strategy with capacity and capability planning, business planning and investment decisions. We plan to being to develop this framework in 2025-26.
Commit to environmental sustainability and achieving net zero
We monitored and reported on our carbon usage and waste and achieved our target to maintain our business travel at 60% of pre-pandemic rates. We also continued to limit our domestic flights and centrally reviewed all domestic flight requests to make informed decisions about the most appropriate form of travel balancing sustainability, cost, access needs and health and well-being. We used just 3 domestic flights this year having reviewed all criteria and confirming these were the best solution for travel.
We maintained low levels of single-use plastics and paper purchases as per the Greening Government Commitment and we incorporated carbon reduction targets into contracts in agreement with suppliers on a case-by-case basis.
We considered sustainability impact in all new or changing activities and policies. We did this by developing and implementing a Sustainability Impact Assessment procedure as well as making sure an environmental sustainability statement is included in all policies.
Finally, we understood better our impact by considering our existing ways of working and how these can be improved to be more environmentally sustainable. We developed an Environmental Sustainability Annual Report which outlines associated governance, strategy, risk management and emissions monitoring.
Sustainability report
The HRA is committed to environmental sustainability and achieving net zero. To help make this happen, the Green Team - a staff-led group committed to improving sustainability - developed and launched our first Environmental Sustainability Strategy in 2022. This strategy aims to embed environmental sustainability as part of our culture and ways of working. This was updated for 2024, and sustainability will be part of the refreshed HRA strategy for 2025.
We have made good progress this year with continued emissions monitoring and increased awareness of our Environmental Sustainability Strategy. We are pleased with how much we have achieved, and want to do more to make sure we play our part ensuring the environment is protected for future generations.
We work in partnership with our colleagues at the Department of Health and Social Care (DHSC) and our Green Team, to improve environmental performance across the organisation. Our approach continues to focus on staff-led initiatives alongside strategic commitments to deliver significant, lasting change. Our vision, to make this change happen, is to embed environmentally sustainable practices into our daily business, making environmental sustainability the norm.
Our work is aligned with the 5 environmental principles, as set out in section 17(5) of the Environment Act, encouraging not only our teams but also our partners and suppliers to consider the environment in all activities.
Our estates strategy also works to support our sustainability strategy. This includes ensuring that sustainability and environmental performance is prioritised in decision making. We also make sure lease events are managed, meeting government policy and supporting our strategy. In 2024, our 5 office locations were in spaces shared with other government bodies to improve office utilisation and energy efficiency.
Where we share offices, energy reporting is provided by our public landlord and is not included in our Greening Government Commitment reporting.
Several initiatives have been introduced to support our strategy, including:
- looking at how we buy goods and services to make sure sustainability considerations are included in decision-making
- introducing an Environmental Sustainability statement in all new policies and procedures
- assessing all domestic and international flight requests against several factors including cost, time, energy consumption and staff wellbeing before booking travel
The following tables show our key performance indicators that help us measure our ambition to achieve net zero.
Carbon Dioxide (CO2) emitted by rail travel – staff
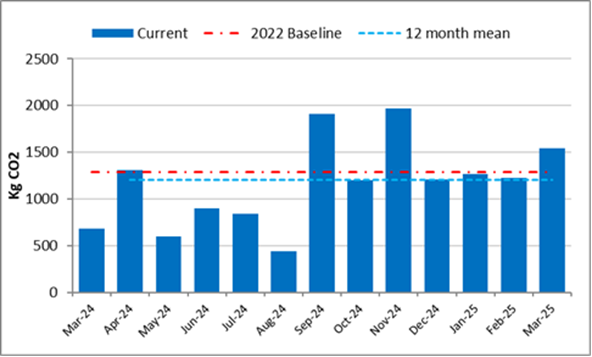
The graph shows monthly carbon dioxide CO2 emitted from rail travel undertaken by staff from April 2024 to March 2025. The 2022 baseline and our target for CO2 is shown in red at just below 1,500 KgCO2. Our staff travel in 2022 was significantly lower than pre-pandemic levels and travel this year was slightly below this target. We set this target as a stretch target for 2024, unsure what our future travel requirements would be following the pandemic. We are closely monitoring our staff travel to set a more realistic target level for next year, using the data from the last 3 years, so we meet both our sustainability goals and make sure our people can work well.
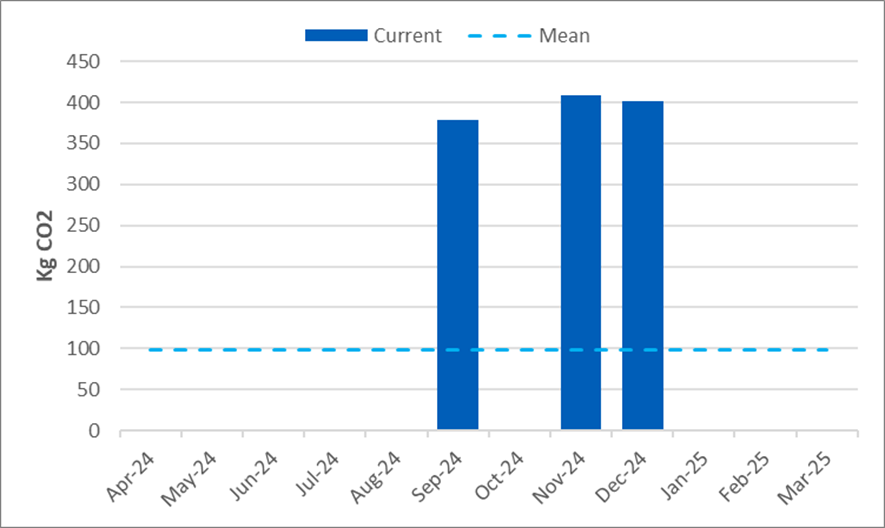
CO2 emitted by rail travel – Committee members and public contributors
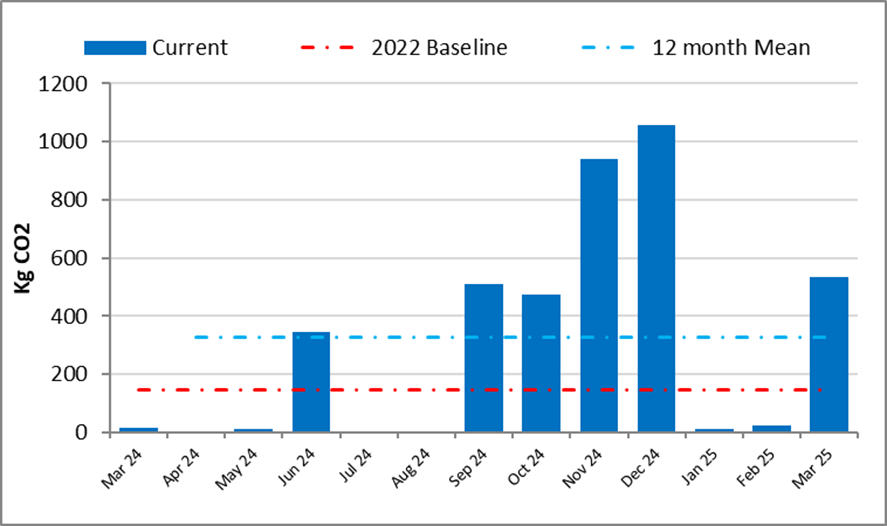
The following graph shows monthly carbon dioxide (CO2) emitted from April 2024 to March 2025 through rail travel undertaken by committee members and the public who are involved in our work. The 2022 baseline and our target for CO2 is shown in red around 150 KgCO2. Again, this level was set as a stretch target and will be monitored closely to help us set a realistic target for future monitoring.
Carbon Dioxide (CO2) emitted by rail travel – Committee members and public contributors
Staff and HRA Community domestic flights – cumulative CO2
There were 3 domestic flights during 2024-25 (2023-24: 2). Each domestic flight was reviewed against agreed criteria to balance sustainability against other factors such as time and traveller's health and wellbeing.
Carbon Dioxide (CO2) emitted by HRA staff and Community domestic flights
Dr Matthew Westmore
Chief Executive
Health Research Authority
11 July 2025