The Over-Volunteering Prevention System (TOPS) is a database, free to all UK organisations undertaking Phase I trials in healthy volunteers, that aims to prevent participants from taking part too frequently in trials of new medicines.
Volunteers should be registered on TOPS when they attend a unit for a screening exam. Once the volunteer is registered on TOPS, other units using the database will be able to see that the volunteer has attended the unit and may be intending to participate in a study.
TOPS provides contact details for staff at other units. If you need more information about a previous registration, you can contact staff at the relevant unit.
TOPS was run by an independent charity, but in April 2013 its function was transferred to the Health Research Authority (HRA). It is a standard condition of ethical approval, as well as part of the Medicines and Healthcare products Regulatory Agency (MHRA) accreditation scheme, that all Phase I studies using healthy volunteers register research participants onto TOPS and complete the record for each volunteer to specify whether they received a dose of the study medicine.
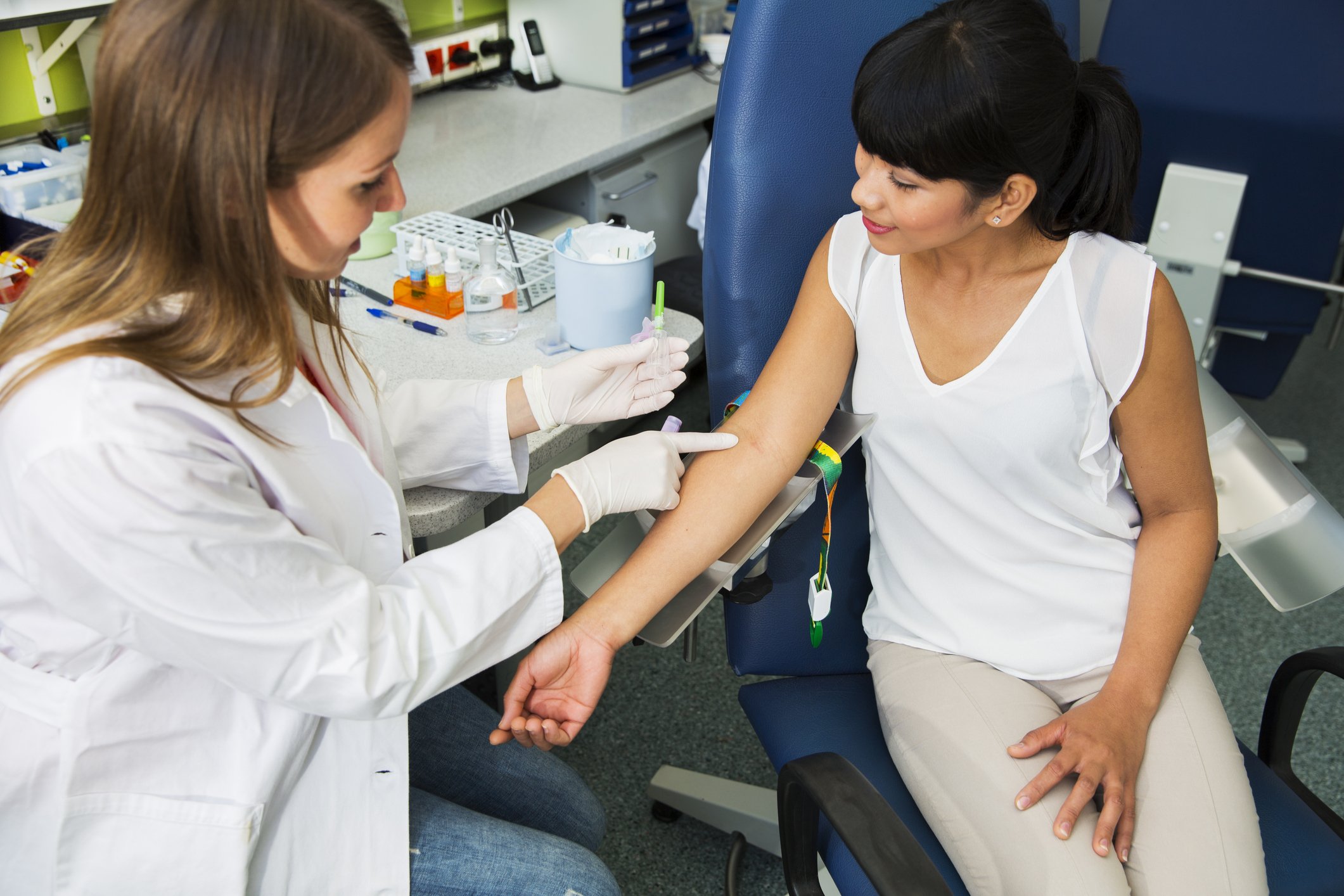
Why do we need TOPS?
Healthy volunteers must not take part too often in trials of new medicines, for scientific, medical and ethical reasons:
- if the gap between two trials is too short, or the trials overlap, the medicines might interact
- taking too many blood samples could cause anaemia
- it’s unethical to expose healthy people too often to medicines they don’t need
How does TOPS help?
TOPS allows volunteers to be identified and for it to be determined when they were last registered to take part in a study.
The database is designed to detect most cases of accidental or deliberate over-volunteering, but it is not designed to detect professional over-volunteering or fraud.
Participant information sheets and consent forms
TOPS users must get the volunteer’s permission to allow them to register the volunteer on TOPS.
This statement is suitable for insertion in the Participant Information Sheet and consent formHealthy volunteers must not take part too often in trials of new medicines, for scientific, medical and ethical reasons:
- if the gap between two trials is too short, or the trials overlap, the medicines might interact
- taking too many blood samples could cause anaemia
- it’s unethical to expose healthy people too often to medicines they don’t need
So to help research units, the Health Research Authority keep a database of healthy volunteers and when they take part in studies, this is called TOPS. We will enter into the database: your National Insurance number (if you’re a UK citizen), or your passport number and country of origin (if you’re not a UK citizen) and the date of your last dose of study medicine. If you withdraw from the study before you receive any study medicine, the database will show that you never received a dose. Only staff at (insert the name of your unit) and other medicines research units can use the database. We may call other units, or they may call us, to check your details. Data entered in TOPS is retained for the minimum period required and this is determined based on whether you receive a dose of the study medicine or not. If you receive a dose of the study medicine, this data will be retained in TOPS. If you do not receive a dose, your data will be retained in TOPS for two years. If we need to contact you about the study after you’ve finished it, but we can’t because you’ve moved or lost contact with your GP, we might be able to trace you through the information in the database.
How to use TOPS
Only registered users can access TOPS. To obtain a username and password for your unit please email: tops@hra.nhs.uk
A user manual is available on the TOPS database, and you can read Frequently Asked Questions about TOPS on our website.
TOPS database responsibilities
Users are responsible for the accuracy and quality of the data entered into the TOPS database.
TOPS users are also responsible for ensuring that data is entered and updated in a timely manner and that data is held securely on their computer.
We are responsible for the maintenance of TOPS and for ensuring that the transmission of data entered into TOPS is secure.
Contact details
If you would like any further information regarding using TOPS, or if you need to make an amendment to TOPS data which has been entered by your unit, please email: tops@hra.nhs.uk